In-Situ Bioprinting – Patent Landscape Study
The earliest tissue engineering evidence dates back to the old Sanskrit texts around 3000 BCE, wherein skin grafting was used to reconstruct facial features. Almost 5000 years later, the term tissue engineering was coined by bioengineer Yuan-Cheng Fung in a proposal to the National Science Foundation (NSF). Ultimately, the publication of the review article titled “Tissue Engineering” in 1993 by Robert Langer and Joseph Vacanti caused a widespread awareness of the technology, which resulted in an exponential increase in research and development.
Back in 2002, realizing that the droplets of ink in a standard inkjet printer are about the same size as human cells, Professor Makoto Nakamura decided to adapt the technology, and by 2008 had created a working bioprinter that could print out bio tubing similar to a blood vessel.
Table of Contents
What is Bioprinting and how is it done?
Bioprinting refers to the fabrication of tissue or other biologically active materials and constructs by developing dissolvable gel scaffolds with the use of an additive manufacturing technology (AM); into which bioink (biomaterials like cells, growth factors, hydrogels, crosslinkers, Fibrinogen, ECM, etc.) is incorporated.
The design of the construct is governed by predefined 3D models that have been scanned from live references and developed in computer aided design (CAD) software and printed using a 3D bioprinter. There are numerous studies and examples of successful application of bioprinted constructs such as prosthetics, stents, various tissues and organs such as bones, cartilage, myocardial tissues, heart valves, blood vessels and trachea using inkjet, laser sintering or extrusion printing technologies.
Limitations of Bioprinting
3D bioprinting comes with its drawbacks. As this method requires developing a functional organ in-vitro environment (using a bioreactor), the parameters have still not been recognised. Moreover, the handling of these fragile constructs is a difficult task, the risk of contamination and rejection by the recipient’s body, incorrect shape and morphology due to low resolution imaging and printing systems are a few of the many challenges faced by researchers working on this field.
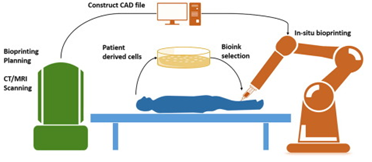
Enter In-situ Bioprinting!
To overcome the aforementioned problems in-situ bioprinting has sparked a medical revolution. In-situ bioprinting, sometimes referred to as “in-vivo” bioprinting is the process of directly printing bioink in the damaged tissue in the human body. One of the crucial advantages of this system is the elimination of an artificial micro-environment for printing as the human body here behaves as the in-vivo bioreactor.
The idea was first proposed by Phil G Campbell and Lee E Weiss of Carnegie Mellon University in 2007 by using inkjet technology.
Current In-Situ Technologies & Bioprinters
Current bioprinting techniques can be divided into three main categories, namely – Droplet-based Bioprinting (DBB), Extrusion-based Bioprinting (EBB), and Laser-based Bioprinting (LBB). The selection is done on the basis of the target organ development. These modalities can be implemented in two different approaches; robotic arm approach and handheld approach assembled with a separate bioprinting unit, which goes into the body through minimally invasive routes and reconstructs the damaged part.
- Recognizing the need and the potential for this technology, 3D printer manufacturer Aerosint and precision laser micromachining specialist LASEA are collaborating to build an additive manufacturing system with in-situ laser ablation aided research grant of €1 million from the regional government of Wallonia, Belgium.
- A “Biopen” is another type of bioprinter that has been developed by Cathal D O’Connell of University of Wollongong for the handheld biofabrication of tissues, which enables the deposition of living cells and biomaterials in a manual, direct-write fashion. However, no in-situ printer has been commercialized for bioprinting in clinical settings.
IP Overview
A patent landscape of the domain revealed that the technology is still protected yet rapidly growing with 30+ patent applications worldwide with around half of these applications been granted. The patents majorly focus on bioprinting systems comprising of bioprinters and stations that are capable of in-vivo printing. Most of the patents focus on robotic or automated systems rather than hand-held systems to improve accuracy. Skin regeneration and tissue reconstruction are the most explored application areas.
Wake Forrest School of Medicine is the top player in the domain with 5 patent applications under their belt. Regenovo Biotechnology is following closely the in-situ bioprinting race with their live skin-repair bioprinting technology. Overall, China is leading the research in bioprinting with the highest number of patents in the domain.
Success Stories
- Wake Forrest School of Medicine has developed a portable bedside skin printer with a team of bioprinting researchers lead by Anthony Alata, partly funded by the US military. They have taken 3D scans of test injuries inflicted on some mice and have used the data to control a bioprint head that has sprayed skin cells, a coagulant and collagen onto the wounds which resulted in rapid wound healing in just two or three weeks.
- Another group of researchers and engineers led by Yusef Khesuani of Moscow Oncology Research Center conducted a unique experiment on implant in-situ bioprinting to replace skin defects. As in-situ bioprinting technology combines surgical robotics with three-dimensional bioprinting tissue grafts, in-situ poses to be a software and computer vision challenge as well.
- A group of researchers from INSERM, France successfully printed endothelial cells using Laser-assisted Bioprinting (LAB) into mouse calvaria bone defects to generate a pre-vascularization network to aid in-vivo bone regeneration.
Risks & Future Prospects
In spite of the rapid technological development and numerous inventions, in-situ bioprinting is still in its nascent stage and it has to overcome many hurdles before entering the operation theatre of hospitals.
Improved bioink has to be developed as most of the current available bioinks require curing before implantation. Printing organs or tissues require a large number of cells along with other biomaterials which require in-vitro cell expansion from the patient, resulting in a lot of ethical concerns. Constructing functional and readily transplantable tissues is a very expensive process, which in turn will only cater to the patients who can afford the treatment. Detailed scanned information of an individual will provide surgeons and corporations with extensive information about an individual which might lead to misuse of information.
This novel research field provides great potential for tissue engineering and regenerative medicine applications where preoperative planning of construct size and shape is difficult or impossible to predict. Together with developments in nanotechnology and genetic engineering, artificial intelligence and IoT, advanced bioinks, improved scanning, advanced software, multiple axis printing bioprinting with high resolution and most importantly, strict regulatory laws may soon prove to be a powerful tool for those in pursuit of life extension. Overall, In-situ bioprinting has the potential to play a major role in organ formation and regeneration.
– Rajarshi Bhowal (Life Sciences Team) and the Editorial Team