Most Comprehensive and Curated Database of References Related to Rare Diseases
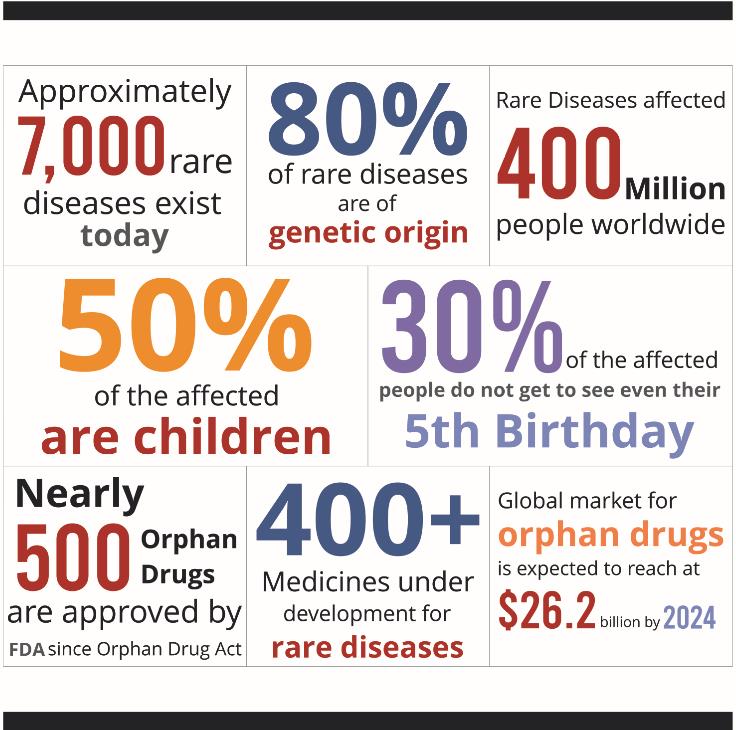
- The rare diseases market is growing faster than the market for traditional drugs (400+ drugs under development)
- Financial Incentives and Research Grants are provided by Governmental and other Non-Government Organizations
- Big companies are placed to buy and market developed drugs if not developing themselves
- And this is leading to increase in overall data related to rare diseases treatment and diagnosis including patent filings, Non-Patent Literature, etc.
Our Motto
Contributing its part in the development of technologies for Diagnosis and Treatment of Rare Diseases by helping innovators of companies, research institutes, to access the already available information for rare diseases, so that the innovators have information readily available for their use with respect to current technologies in the domain and therefore a better Return on Investment is achieved for the overall development of drug/ treatment/ and technology for diagnosis. The information would help innovators to innovate with confidence, and convert emerging opportunities into market leading products
Salient Features
- Data curated by team of technical experts with expertise in IP Analytics
- Interactive Database providing filtered information for data (patents, non-patents, clinical trials) related to various rare diseases
- One Click access to patents and non-patents based on the technicalities
- Account management for restricted access at different levels
- Option to share important references within team through different mediums such as Emails, Microsoft Teams etc.
- Quick Analytics providing data in graphical format for easy consumption of information
- Easily available information for decision making
How this Helps
Through this database, we are supporting the innovators and decision makers at various levels, by providing them access to filtered information related to different technologies developed and/or are under development, which is useful for them to save their resources, and spend the grant money majorly on development of technologies (without re-inventing the wheel) rather than on collecting the data of already published technologies.
Pricing
Basic
Premium
Filtered Dataset - as a complete list
✔
✔
First Level Division of Patents
✔
✔
Patents further filtered based on technical heads
-
✔
Basic Visualizations - Filing Trend, Top Assignee, Major Geographies, Division of patents based on first level
✔
✔
Extensive Graphs based on further technical analysis
-
✔
Access to one detailed report per year
-
Available as an Add-on
Free 10 Hours discussion with our analysts
-
✔
Estimated Subscription Cost (per user)
US $1,699 per year
US $2,499 per year
Pricing
Basic
- Filtered Dataset - as a complete list
- First Level Division of Patents
- Basic Visualizations - Filing Trend, Top Assignee, Major Geographies, Division of patents based on first level
Estimated Subscription Cost (per user): US $1,699 per year
Premium
- Filtered Dataset - as a complete list
- First Level Division of Patents
- Patents further filtered based on technical heads
- Basic Visualizations - Filing Trend, Top Assignee, Major Geographies, Division of patents based on first level
- Extensive Graphs based on further technical analysis
- Free 10 Hours discussion with our analysts
- Add-on: Access to one detailed report per year
