The Need for Trademark in Pharmaceutical Industry
Pharmaceutical trademarks are similar to conventional trademarks, with the exception that they are typically registered for reliable pharmaceutical goods and services, such as life-saving pharmaceutical drugs and healthcare products and services. Pharmaceutical trademarks are particularly important since they are directly linked to a matter of public health.
This article is a comprehensive discussion on what you need to know about trademark in the pharmaceutical industry, their importance, why pharmaceutical trademarks are needed, and how to register a trademark in pharmaceutical industry.
Table of Contents
What is Pharmaceutical Trademark?
Pharmaceutical trademarks are mostly filed for reputable pharmaceutical goods and services. In the case of pharmaceutical trademark, the brand name or drug name is typically taken from the drug’s treatment, salt composition, or any other associated medical word. For example, if the drug for the liver is called LIV, the next drug from the same firm will be labeled “LIV 1.”
Medicinal and pharmaceutical products are commonly named after their generic ingredients. However, such marks are frequently regarded as weak or non-distinctive because they can easily be traced back to their individual ingredients.

Importance of Pharmaceutical Trademark
Pharmaceutical trademarks are important because they are directly related to public health issues and help users identify the products they want, which improves the reputation and motivates pharmaceutical companies to maintain high quality. By minimizing name-related prescription errors, pharmaceutical trademarks protect a patient’s health and safety. Any chance for error or mistake in a medicine’s nomenclature, such as identical sound or resembling names or any similarity in trade style, packaging, etc., might affect a patient’s purchasing decision, whereas the purchase of the wrong drug can be fatal. As a result, courts are strict in this regard and take particular caution when evaluating problems related to the pharmaceutical industry, with public welfare and health as the highest priorities.
Due to business and regulatory factors, the pharmaceutical industry’s procedure is extremely time-consuming. Here’s a quick checklist of actions you must take with the help of an IP professional or trademark attorney to help you register an effective pharmaceutical trademark:
- Make a list of possible trademark contenders and rank them in order of importance.
- Proceed with the strongest options; conduct a full trademark clearance similarity search to determine the risk of your trademarks being confused with existing brand names.
- Proceed with the strongest options; conduct a full trademark clearance similarity search to determine the risk of your trademarks being confused with existing brand names.
- To keep track of possible infringement by using both traditional and non-conventional resources.
- File for trademark registration.
Manufacturers might also be enticed to invest in new treatments by using trademark to secure the safety of their existing drugs. Apart from this, trademark in the pharmaceutical industry also:
- Supports Health Professionals to Minimize Medication Errors.
- Enables Consumers to Choose Suitable Medications.
- Allows Manufacturers to Monitor Their Drugs.
Naming a New Pharmaceutical Drug
Pharmaceutical products are distributed all over the world. Thus, pharmaceutical companies must choose a trademark that is recognized in every country where the product will be sold. Companies who are introducing a new pharmaceutical product must conduct a global trademark search to ensure that their intended trademark is not already in use and that their branding does not have a negative impact in particular regions. Before attempting to sell a pharmaceutical product, it is critical to seek approval from the appropriate health authorities as well as the appropriate trademark registries.
There are multiple names for all pharmaceutical drugs. This includes a scientific name, which follows International Union of Pure and Applied Chemistry (IUPAC) rules for small molecular medicines. Because these names are often long and complex, a nonproprietary or generic name is also chosen. These names must go through an approval process to make sure they do not sound too similar to existing drugs and that the same ‘stems’ aren’t used to name related chemical compounds. As a result, drugs that treat a certain type of condition end with the same suffix (e.g., ‘-lol’ for beta-blockers).
Naming Process
The Ultimate goal is to have a name that:
- Satisfies branding and marketing goals.
- Minimizes the risk of medication errors.
- Is approved by the United States Patent and Trademark Office (USPTO) and foreign trademark offices.
- Is acceptable to the FDA, EMA, and other regulatory bodies.
- Name ready and approved by launch day (timing is important).
Check Similar-Sounding Pharmaceutical Trademark
The similarity in drug names should be avoided since the possibility of confusion endangers consumer health. Bringing the greatest pharmaceutical trademark to market can be challenging, but it will help with medicine safety. Confusion for similar-sounding pharmaceutical trademark is influenced by similarities in design, packaging, or pattern. Using these pharmaceutical marks in a way that is likely to deceive customers, as with any trademark, jeopardizes goodwill, affects fair competition and may result in serious health risks for consumers.
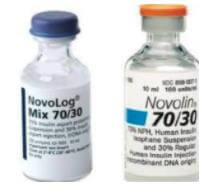
For Example: – The major difference between these two insulins is that Novolog 70/30 contains both intermediate and very fast-acting insulins, while Novolin 70/30 contains both intermediate and short-acting insulins.
Regulatory Approval in Pharmaceutical Branding
After the selection of suitable names for a new drug or product, pharmaceutical companies must secure regulatory and legal approval, which can be highly complicated and time-consuming. Pharmaceutical products must be approved by a health authority and registered with the appropriate trademark before they can be sold on the market. Regulatory approval allows you to sell the pharmaceutical drug under the approved brand name. Hence, pharmaceutical companies must select strong trademarks that are available in each country where the product will be introduced. Before registering a pharmaceutical trademark, a global trademark search must be conducted to guarantee that the desired trademark is unique and that the branding does not negatively affect any country. The following aspects can aid in the selection of a strong pharmaceutical trademark:
- The rule of the standard trademark should apply to pharmaceutical trademarks.
- The pharmaceutical trademark must be registered with the USPTO.
- The guidelines of the United States Food and Drug Administration (USFDA) should be followed when creating a pharmaceutical trademark.
Foreign Direct Investment (FDI) in Indian Pharmaceutical Sector
- Pharmaceutical Sector is one of India’s top ten industries attracting foreign investment. In Medical Devices, 100% foreign investment is permitted under the automatic approach, wherein foreign investment in pharmaceutical greenfield projects is authorized up to 100%, and foreign investment in brownfield pharmaceutical projects is allowed to invest beyond 74% to up to 100%. But approval from the government is necessary.
- After the fall of the Foreign Investment Promotion Board (FIPB) in May 2017, the Department of Pharmaceuticals was tasked with reviewing foreign investment proposals under the Gov-11 Annual Report 2020-21 government approval procedures. Besides that, the Department analyses any pharmaceutical FDI proposals arising from Press Note 3 of 2020, dated 17.04.2020, in which the investors/ultimate beneficiaries are from the land-sharing bordering countries of India.
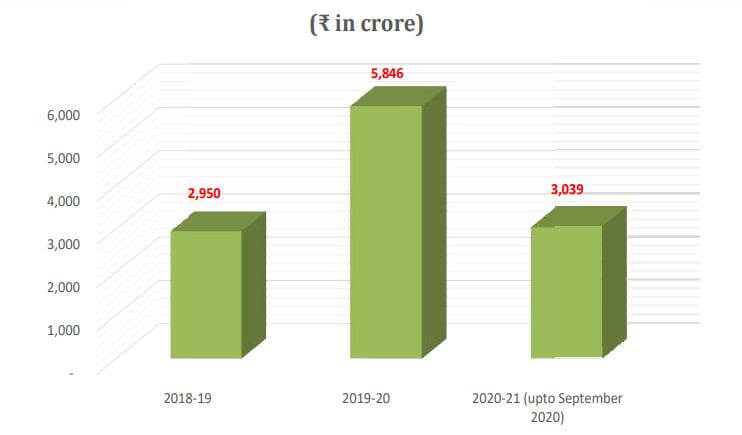
- Between April 2020 and December 2020, the Department of Pharmaceuticals approved 17 FDI bids for brownfield pharmaceutical projects worth Rs 1,512 crore.
Advantages of Trademark in Pharmaceuticals
Some of the major advantages of Trademark in Pharmaceuticals are as follows: –
1. Allowing Drug Manufacturers to Monitor Their Products
Manufacturers will be able to monitor the safety of their existing drug, thanks to the use of trademarks. Without the use of a trademark, they would be unable to determine whether their products were involved based on reports of side effects that just stated the drug’s common name. It would result in costly investigation that may be avoided if the manufacturers involved were distinguished by Pharmaceutical Trademarks.
2. Encouraging Health Professionals to Prevent Medical Error
The purpose of the Trademark is to ensure that the mark is unique and original. Due to the similarities of multiple generic names for pharmaceuticals in the same category, many nurses, physicians, and other practitioners find it difficult to recall or spell common names. Healthcare practitioners ensure that the correct medication is administered to patients by employing unique Trademarks for each pharmaceutical drug.
3. Letting users choose suitable medications
People are typically hesitant to switch from their favorite branded drug to a generic equivalent. The peace of mind that comes with the administration of a well-known branded drug may be the reason for some clients to exercise brand loyalty. Users can also learn about the availability of unique treatments for their health conditions quickly, thanks to the start of direct-to-customer advertising in many countries.
How to Register a Trademark in the Pharmaceutical Industry
While all potential trademarks face similar problems in terms of creation and registration, pharmaceutical companies that want to protect themselves with a trademark confront a number of unique issues, particularly with respect to Pharmaceuticals. Pharmaceutical trademark approval and protection can be difficult since brand owners and IP specialists must not only design a wholly different brand name, but must also guard against counterfeiting, trademark infringement, and passing-off attempts. Any new naming stage always starts with a big list of possible names, which is narrowed down to more reasonable proportions using a range of approaches. Some companies will conduct a deeper comprehensive search to eliminate names that may be confusingly similar to existing trademarks, as well as to reject names that may have unexpected meanings or implications in any of the languages spoken in the areas where the product will be sold.
Comprehensive trademark searches guided by Sagacious IP experts allow you to confidently kick start your final pharmaceutical names. Our evaluation for your final-candidate names combines the global trademark registration data with name safety to ensure that any potential conflicts have been detected and reported for your final inspection. For more details regarding the trademark registration, click here.
Non-Conventional Trademark
In today’s age of competitive marketing, a variety of tactics are used to capture consumers’ attention. Non-traditional trademarks are also playing a role in the same. Non-traditional trademarks exist beyond the scope of standard trademarks. These include marks based on shape, sound, smell, taste, and texture. In recent years, pharmaceutical businesses have started using advanced and novel strategies to differentiate their products from those belonging to competitors. As a result, pharmaceutical companies are depending on non-traditional trademarks to stay ahead of the curve, in addition to merely the brand name or drug name. This helps in avoiding confusion among customers while simultaneously emphasizing the trademark’s uniqueness.
In terms of non-traditional trademarks, India has a long way to go. With the introduction of new technology and developments in trademark recognition and association, going beyond the old approaches has become vital.
Trademark Infringement Cases In the Indian Pharmaceutical Industry
In the Indian pharmaceutical industry, there have been a bunch of cases over the years involving pharmaceutical trademark infringement. One common feature of these several cases is that the risk of misunderstanding in cases involving drugs must be kept to a minimum, if not zero, to avoid any negative impact on public health. Some well-known trademark infringement cases in the Indian pharmaceutical industry are discussed below:
Case 1: Cadila Healthcare Ltd. V. Cadila Pharmaceutical Ltd, 2001(5) SCC 73
Cadila Healthcare Ltd. was granted a trademark for “FALCIGO” to market its medicine for cerebral malaria. After a year, Cadila Pharmaceuticals Ltd. launched their drug “Falcitab” in the market. Cadila Healthcare Ltd. filed a complaint in the district court of Vadodara seeking a judgment prohibiting Cadila Pharmaceuticals Ltd. from using the term “Falcitab. ” According to the appellants, both pharmaceuticals were used to treat the same condition, the labels were misleadingly similar, and both medicines were to be prescribed as a last resort. But according to the responders, the word ‘Falci’ is derived from the disease Falcipharum malaria and is used in the pharmaceutical sector.
Despite the fact that both pharmaceuticals can only be bought through a prescription, the Supreme Court concluded that this information was insufficient to prevent confusion. The Supreme Court declared that the disturbing similarity criteria must be amended in the case of pharmaceutical medications since misreading medicinal products can be fatal. As a result, the injunction was considered to be appropriate in this case. The Supreme Court of India also set the following factors for determining whether two pharmaceutical trademarks are deceptively similar:
- The type of trademarks, such as a word, label, or composite mark.
- The degree to which the trademarks are similar.
- The kinds of goods or services for which they are used as trademarks.
- The similarity in the character, nature and performance of the rival’s products.
- The type of buyer who is most likely to purchase or use products with the trademarks they need, based on their education and intellect, as well as the degree of caution they are likely to practice while buying or using the products.
- How things are purchased or orders are placed.
- And any other information that could help determine the degree of dissimilarity between the competing marks.
Case 2: Mankind Pharma Limited V. Novakind BioSciences Private Limited
In the case of Mankind Pharma Limited v. Novakind BioSciences Private Limited, the Honorable High Court of Delhi granted an ex-parte ad-interim injunction in favor of the plaintiff (Mankind), prohibiting the defendant (Novakind) from, among other things, manufacturing and selling any pharmaceutical product with the suffix “KIND.” The plaintiff was the owner of the well-known trademark “MANKIND” and a number of other marks with the suffix “KIND,” while the defendant was using the mark “DEFZAKIND” for its pharmaceutical medicine.
Case 3: Cipla Limited vs. M.K. Pharmaceuticals MIPR
In this case, the plaintiff sold “NORFLOXACIN” tablets in blister packaging that were oval in shape and orange in color, under the trademark “NORFLOX-400.” The defendant adopted the identical name, “NORFLOX-400.” However, the plaintiff did not sue the defendant for the name; rather, the plaintiff alleged that the defendant imitated the shape, color, and blister packaging of tablets, causing confusion. It is well understood that no color monopoly can exist as no one orders medicine based on its color, form, or packaging. The use of blister packaging is common, and pills are frequently round or oval in shape. As a result, there was no injunction issued.
Case 4: Neon Laboratories Ltd. V. Medical Technologies Ltd.
The appellant filed a trademark registration application in 1992, and was granted the trademark “ROFOL” in 2001. However, the product was not released until 2004. Meanwhile, in 1998, the respondent introduced the drug “PROFOL.” Due to this, the respondent had an earlier user date, and the appellant had an earlier registration date. The case was in favor of the respondent as the respondent began producing and distributing, as well as earned substantial goodwill in that period. As a result, the trademark “ROFOL” was placed under injunction. The temporary injunction factors of balance of convenience and irreparable damage favored the respondent.
Why Choose Sagacious IP?
Since trademarks are valuable in the pharmaceutical industry, it is necessary to do a comprehensive search before registering one to avoid duplication. The trademark search is required to avoid the infringement of a drug manufacturing formula, as each drug takes extensive study and has its own manufacturing formula. Whether you’re looking for a trademark in the USPTO database, the WIPO database, the EPO database, or a custom search, Sagacious IP’s experts can help you find what you’re looking for. Some of the reasons why Sagacious IP is the best option for you are as follows:
- Dedicated team of 20+ Trademark experts.
- Trademark searches on global, cluster-based, and local level.
- Find existing mark names that are Exact, Near Exact, or Similar Sounding in the same Classifications and Goods & Services.
- Find all possible identical brand/trademark names that could jeopardize the trademark rights.
- Find out about any other well-known brand names or trademarks that are comparable.
- Make a list of restricted trademarks so you can see if their brand name or trademark is included in the list.
- Identify other products/logos that appear to be similar to respective companies.
- The team has all of the tools necessary to offer you precise and dependable data.
- Solutions that is both affordable and effective.
- Excellent Results.
- Flexible Delivery Schedules based on Customer Needs.
Conclusion
After examining pharmaceutical Trademarks, it is clear that Trademarks are important in the pharmaceutical industry. To avoid trademark infringement, a systematic search should be done prior to registration to avoid similar naming and sound-alike pharmaceutical names, whether you’re searching the WIPO Trademark Database, the EPO Trademark Database, or conducting a custom search. A pharmaceutical trademark identifies a drug manufacturer’s success by prohibiting counterfeiting of its products/processes. To summarize, Indian courts are adopting a stricter approach to adjudicating the probability of confusion in pharmaceutical trademarks due to the impact on human life. As a result, based on the opinions adopted by various courts, the following aspects should be considered when selecting a brand name for pharmaceutical products:
- Avoid phrases or words that have become familiar in the business or are descriptive, as they may not function as source identifiers but rather as markers of ingredients or diseases.
- Avoid suggestive words or terms.
- Avoid the danger of infringement or passing off action, and conduct a check on the Trade Marks Registry website before registrations or applications.
- Conduct a google search to see whether there are any similar pharmaceuticals on the market, and avoid names or taglines that are similar in appearance or sound, as well as trade dress or packaging that is comparable to your goods.
- Avoid terms or marks that may be misleading because they contain inert or inactive components.
Sagacious IP’s comprehensive Pharmaceutical Trademark Search solutions are designed to provide the customers with the information they need before filing for trademarks. The experts at Sagacious IP can assist you in achieving your goals. The team has every resource at its disposal to give you reliable and accurate data. Furthermore, we assist clients in identifying existing and available trademarks for registration using our Trademark Search service. More information about this service can be found by clicking here.
– Priyanka Nimje (Trademark) and the Editorial Team
Having Queries? Contact Us Now!
"*" indicates required fields




