How to Conduct Effective Patent Licensing Studies in Life Sciences Domain
Those operating in Pharmaceutical and Healthcare domain are well aware that the cost of research and development in the sector is relatively higher than other areas. It is accompanied by increased risk in terms of Return-On-Investment (ROI). That said, patent licensing emerges as a favorable option for companies in these domains that are figuring out options to invest and associate with already existing technologies instead of manufacturing from scratch. The following article sheds light on how companies can conduct licensing studies to formulate effective and rewarding patent licensing strategies.
Table of Contents
Patent Licensing Studies and Why It is Needed
Regarded as one of the effective methods of patent monetization, licensing is opted by businesses that are looking to supplement revenue generation to their business. In the life sciences domain, there are certain challenges associated with the monetization of patents. These include compulsory licensing, a long drug approval process and high R&D cost, which make the licensing process complex and challenging. So, licensing studies can be helpful in overcoming these obstacles. Some of the scenarios wherein licensing studies are beneficial are:
- Gaining exclusivity rights before approvals
- Patent pooling
- IP Audits during business strategies
- Drafting royalty/negotiations
Conducting Licensing Studies in Life Sciences Sector
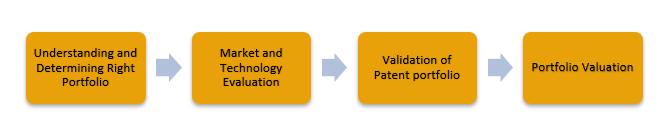
1. Understanding and Determining Right Patent Portfolio: This step is a fundamental part of any licensing study.When companies have a rich patent portfolio, they determine its ranking while considering licensing. It is based on bibliographic and qualitative parameters. While the former parameters include factors like IPC, citing and cited references; the latter comprises similarity of the cited reference to the subject invention. These licensing studies use a patent ranking algorithm that analyses patents based on parameters like quality. Thereafter, these parameters are arranged in an order to achieve various business objectives. After these parameters are studied, top patents from the perspective of licensing and sales are identified.
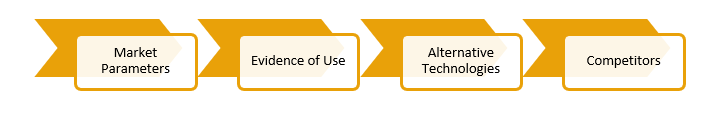
2. Market and Technology Evaluation: Under this process, a comprehensive evaluation of market and IP research is carried out for the respective and competing technologies. The process includes reading product descriptions, brochures, scientific literature, patent grants and applications. All these documents are studied in detail, based on which, market scenario, investments, partnerships, and top competition are identified. The parameters considered while conducting the market and technology evaluation are:
3. Patent Portfolio Validity Check: Along with the other mentioned steps, a portfolio validity check is performed to determine prior-arts that can attack a portfolio’s novelty and non-obviousness or literature that may challenge its claims. It helps applicants to understand a higher likelihood of competing prior-art, if any. If yes, the valuation of the patent gets affected. The applicants of the competing prior-art, identified during the study, can often be product specific competitors or potential licensees.
4. Patent Portfolio Valuation: Traditionally, such a study includes either of the two approaches – the market approach and the IP evaluation approach. The former involves studying market size and market share of the technology to conclude the valuation based on potential market impact. On the other hand, the latter involves calculating the annual turnover of the patented technology. At Sagacious IP, patent practitioners use a hybrid approach involving both market and IP evaluation for more reliable results.
Placing Methods Into Practice – A Case Study
Sagacious IP was recently approached by a Fortune 500 client who had a portfolio of around 500 patents. The client wanted Sagacious IP’s support in order to exploit its vast portfolio to its full advantage – in terms of monetization.
Sagacious IP’s first approach was to perform patent pruning to determine the most relevant patents that the client could focus on for evaluation. Herein, almost 500 patents related to the technology were studied and ranked according to key parameters. The filtering process helped in shortlisting top 10 ranked patents that focused on the client’s core technology of interest.
Thereafter, the team of analysts performed a competition spotting study to identify the leading competitors. A detailed analysis was performed to deliberate upon how those companies could be used by the client in terms of their market, products, collaborations, and technologies. The competitors were also ranked in terms of revenue and technology application areas.
Finally, the client was provided with a review-summary, based on this ranking. The client could use this to finalize top potential licensees they could sell their patents to. As a result, the client was able to clearly identify their most relevant patents and comparative technologies. The licensing study helped the Fortune 500 company to formulate an effective patent licensing strategy to fully leverage their patents.
Conclusion
Patent licensing studies are useful for businesses during any phase of the company’s lifecycle. Be it a start-up wanting to determine its IP worth, or a large corporate looking for monetizing patents; all businesses can use such studies to fully leverage their patent portfolio. Similarly, when a company has a vast patent portfolio, licensing studies can help in streamlining other monetization strategies.
Sagacious IP offers end-to-end licensing support to businesses. Our experienced patent practitioners offer the best insights to companies in terms of ranking their patent portfolio and identifying potential licenses, based on which they can formulate their licensing strategies. Click here to know more about our service. To watch a webinar on the subject, click here.
- Devika Saini (Life Sciences) and the Editorial Team
Having Queries? Contact Us Now!
"*" indicates required fields




