Why Patent Prior Art Search is Crucial in Pharmaceutical Literature for Effective IP Strategy
Patent protection for products has always been more significant in the pharmaceutical industry as compared to other industries. The pharmaceutical literature deals with products that humans directly consume. Therefore, there are stringent regulations surrounding the products and the drug approval process.
When a pharmaceutical product is discovered and finalized, a patent is filed. However, despite filing for a patent, the product cannot be marketed before the approval. Due to this long gap between the filing and marketing the product, pharmaceutical manufacturers get shorter periods of patent exclusivity as compared to other patent dependent industries. Further, patents are not usually licensed to other individuals or companies in the same literature because these companies want to enjoy exclusivity in the market and get a return on their investment.
Table of Contents
Prior-Art Searching in Pharmaceutical Literature: An Overview
Prior-art searching in pharmaceutical literature, also known as a patent search, determines the novelty of an invention and identifies existing knowledge related to the product. This search covers both patent and non-patent literature such as published materials, scientific papers, research articles, etc. Patent prior-art search in pharmaceutical literature are unique due to the vast amount of information in the patents and the complexity of this data.
For instance, Markush structures are broad chemical structures that can cover millions of possible structures within one single backbone. Therefore, prior-art searching in pharmaceutical literature becomes tedious and tricky.
When to Request Prior-Art Searching in Pharmaceutical literature?
There are multiple stages at which the patent prior art search can be requested.
- The first stage can be before planning research and development. This is done in the form of a patent landscape to assess the work that has been already done and also to ensure that the research that the company is going to conduct is not a duplication of already existing research.
- The second stage for conducting a patent prior art search can be before filing a patent. This is usually conducted to identify closely related literature and is highly recommended to identify any literature that can be a prior art to the company’s patent. This helps the company in devising the answers to the examiner’s questions and rejections and also helps them work around the existing prior-art.
- The third stage for conducting a patent prior art search is just before the product launch i.e. after the product is completed and is ready to be marketed. Freedom to Operate (FTO) search is usually conducted to identify any patent that this product could be infringing.
- After the product is in the market, there can be a fourth stage when your product is already infringing a patent. In this case, opposition is an option where the patent that is being infringed on can be opposed by searching patent prior art search in literature to invalidate the claims of novelty or obviousness for the particular patent.
FTO Searches in the Pharmaceutical literature
An FTO search specifically in the pharmaceutical literature can be conducted at various stages of the product lifecycle. It can be conducted before the approval of a research project, i.e. the ideation stage, however the most recommended stage to perform these searches is after the completion of drug development and before marketing.
Notably, the FTO searchesat this stage can be used to identify the most relevant references that the product could potentially infringe. Further, FTO searches could be performed during mergers & acquisitions (M&A) or financing or licensing agreements to assess the invention in question and be ware of any potential litigation that the portfolio might attract.
Patent Timelines
For FTO searches in the pharmaceutical literature, timelines can be tricky as compared to other literature as patents for drugs usually get extension periods, such as Patent Term Adjustment (PTA) and Patent Term Extension (PTE) in the US and Supplementary Protection Certificates (SPCs) in Europe. Therefore, an overall timeline for a patent in the literature can be more than 20 years. This information can be obtained from the Orange Book or the USPTO and should be considered while designing an FTO search in the literature.
Best Practices for Prior-Art Searching in Pharmaceutical Literature
1. Keyword-based searches
There are various considerations and best practices for performing patent prior art search. First let us discuss the keyword-based searches that are performed in the literature. The first step of performing a keyword-based search is the preparation of term sets. Once a technology is in hand, keywords or concepts that are important for the technology are extracted. Then, the term sets are prepared using synonyms or the keywords to identify maximum number of patent literature.
In case of chemical formulation or a pharmaceutical formulation composition, four aspects are considered: The first aspect is the active ingredient or the agent (chemical compound keywords), second aspect is the broader concept of the active agent (broader concept keywords), third aspect is the excipient or adjuvant in the formulation that may or may not be playing a very important role (excipient keywords) and the fourth aspect is the application i.e. the indicationthat is targeted by the formulation (application keywords). Therefore, for each of these aspects, keywords are identified, and exhaustive term sets are prepared.
Case Study – Keyword-based searches
In case of Ribociclib which is an anti-cancer agent, first the chemical compound itself – i.e. Ribociclib, its trade name and other names by which it might have been registered for clinical trials are considered. The IUPAC name and CAS number are also taken into consideration as these are unique to every chemical compound. Further, the variations of the synonyms or near synonyms of the IUPAC name are also extracted. All this information is extracted from the sources like PubChem or ChemSpider.
The second aspect is the broader keyword. As Ribociclib targets Cyclin D1 or CDK4/6, patents that would disclose the information in the form of a kinase inhibitor have to be captured. Another way to create broader chemical names is to use the broader chemical category or the backbone of the chemical compound. In this case, pyrrolopyrimidine is the backbone or the chain of the Ribociclib. Therefore, such terms are considered that ensure pyrrolopyrimidine derivatives are also captured in the searches.
The third aspect is the application. Since Ribociclib is used against breast cancer, all terms for breast cancer are included. Also, it is useful to make the indications terms broader i.e. include terms like cancer, tumor, etc. to ensure that everything on indications are captured. Lastly, the other components such as excipients can be used to prepare term sets, if they play an important role in the formulation.
In this example, if the excipients are micro particles, using excipient terms like micro particles and polymers can be helpful. Polymers should be considered as part of the term sets as they are being used to create the micro particles.
2. Classification-based searches
Another strategy that is employed for identifying the references is the use of classification-based searches. The first step in class-based searches is to identify the IPC or CPC codes. This can be identified based on different aspects, which are similar to keywords, i.e. the functional groups, the target of chemical compound, the indications, or excipients. For instance, in Ribociclib formulations, for the functional group or the backbone related classes, we identify a class which considers the heterocyclic ring. Since Ribociclib is an anti-cancer agent, we identify classes related to its antineoplastic properties. This formulation is in an injectable form, therefore the class related to its type of dosage form is also included. Lastly, excipients are also included since they are important.
After identification of important classes, we can use them in various ways by combining them with keywords, or even combining two different classes. However, it is always preferable to use a set of keywords and a set of classes which are of two different aspects. Therefore, an overall holistic approach can be used to search these patents. In the above case, we have taken the class for indication and then used the term sets for compounding. Similarly, various strategies can be developed using the term sets and the IPC or CPC classes.
3. Additional searches
Apart from keyword-based and class-based search, there are also other searches like assignee-based searches that identify the assignees in the literature. Inventor-based searches are employed in the pharmaceutical literature to identify inventors, research groups or labs that are working on specific indications. Further, citations searches have always been an integral part of patent prior art search. They identify closely related prior arts that are not captured via keywords/classes, especially patents in other languages/old references and patents with broad inventions.
Some of the popular sources used by Sagacious IP for keywords, for classes, for assignees and other drug related information include PubChem, ChemSpider, DrugBank, Espacenet, Google Patents, Orange Book, The Merck Index, etc. Further, research papers are another crucial resource that must be used at every stage while developing Term Sets and analyzing references to understand the patents and the information disclosed in them.
4. Structure-based searches
In the pharmaceutical sector, the chemical structure or formula of a compound is essential for patent searches. Structure-based searches are commonly carried out in databases like STN, where the chemical structure of a compound is used as a query to find similar patents. There are three primary types of structure-based searches.
- Exact Structure Search – This search uses the query compound to find patents with identical structures. However, it doesn’t capture related forms like salts or polymers, making it ideal for novelty or invalidity searches but limited in scope.
- Substructure Search – This popular search identifies not only exact compounds but also derivatives like salts, hydrates, and other variations. It also includes polymers if the monomer is indexed, offering a broader search.
- Similarity Search – A broader search type, this looks for structures with similar motifs, used when a wider range of compounds is needed.
Case Study – Structure-based searches
The process involves three steps: first, create a query or chemical structure in software like ChemDraw; second, use substituents to define the search scope, such as determining whether an R substituent is methyl, alkyl, or hydrocarbon.
Once we have performed the search, we need to optimize the search. This can be done by scanning the sample search performed on STN database. And once we have scanned through the references, we can identify what kind of references are being captured. If the number of hits is too high, then we can restrict specific substituents.
Let us understand the optimization & scope with the help of an example – Aryl benzothiazole derivative.
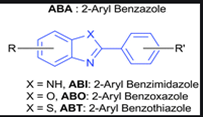
In case we need a broad query structure, i.e. we need not only benzothiazole derivatives, but also closely related structures, X is left open. Thus, X can be anything from -NH, -O to S and depending on what X is, the compound can change as can be seen in the figure.
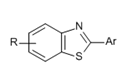
In case of narrow query structure, i.e. we only need Benzothiazole derivatives only, X is not left open. In fact, it is restricted as an -S only. As a result, all the references that are captured are Benzothiazole derivatives.
Other substituents can similarly be left open or be restricted based on the scope of the search similarly.
Keyword-Based Analysis in Pharmaceutical literature
In pharmaceutical patent searches, keyword-based analysis plays a crucial role, especially when dealing with complex drug formulations, chemical structures, and delivery systems. The following components should be considered when conducting a keyword-based search:
- Chemical Salts/Forms – For chemical drugs, patents often cover variations like salt forms, isomers, or crystal forms, which can affect FTO searches. Salt forms influence the drug’s properties without altering its pharmacology. For example, different salt forms of quinapril were protected in one patent claim. Therefore, considering all salt forms is essential in the search and analysis.
- Excipients – While excipients generally don’t impact drug composition, they can play a role in efficacy. For instance, glycerol may act as a preservative. In a patent claim, a composition involving prolactin and ascorbic acid showed that excipients can affect drug efficacy and should be included in the analysis when relevant.
- Dosage Form – The formulation type can significantly affect a drug’s bioavailability and pharmacokinetics, making it an important factor in patent analysis.
- Route of Administration – Drugs can be delivered in various forms, such as pills, powders, liquids, or inhalers, through routes like oral, nasal, or intravenous. For example, a patent on a solid oral insulin dosage form highlights how the administration method is crucial, as insulin is usually injected due to stability issues.
- Delivery System – When a drug’s delivery system, including vehicles and adjuvants, is central to the technology, it’s important to include it in the search. For example, a delivery system using a polymeric hyaluronic acid vehicle for bevacizumab would be vital to analyze.
- Product Synthesis – The methods, reagents, and techniques involved in drug synthesis are key to understanding the technology in a patent.
- Downstream Method Steps – Details about treatment modes, dosimetry, and side effects are essential for comprehensive analysis in some cases.
Evaluating the Relevance of FTO and Landscape Search Results
FTO Search Results
- In case of an invention claiming a formulation comprising of A + B + C + D, any claim comprising the elements A + B + C would be considered relevant.
- Excipients present in formulations are not taken into consideration while evaluating the relevance.
- Claims covering only one of the components of the invention formulation are not a direct threat to the technology. Thus, they are not considered as relevant but as potentially relevant and can be further analyzed to assess the risk.
- Claims having additional components in combination to the pharmaceutical drug formulation are not considered relevant in case of FTO. However, in case of landscapes, combination with additional active agents or having other applications may be considered relevant as the scope of landscape is wider.
- Broad claim covering the concept of the invention, but not specific aspects of the invention are generally not considered as relevant but may be considered as a potentially relevant. For example, in case of search for Ribociclib, the references disclosing kinase inhibitors broadly may not be considered relevant but potentially relevant.
Landscape Search Results
In case of landscapes, broad claims are usually considered non-relevant. Further, the differentiating aspect of landscapes from FTO is that combinations or other applications may be considered relevant.
Case study – Evaluating Reference
Now that we have covered the differentiating points between FTO and landscape, these points can be easily explained using an example of a pharmaceutical composition. Consider a pharmaceutical composition comprising of A + B + C + D wherein A, B and C are baclofen, sorbitol and naltrexone and D is the excipient. As per the analysis of this composition:
- Any patent claiming baclofen, sorbitol or naltrexone will be considered relevant.
- The excipient will not be considered for analysis as it is regular additive.
- Patents claiming only baclofen or sorbitol, or naltrexone will be considered potentially relevant as they do not pose a direct threat to the technology.
- Apart from the three active agents (i.e. baclofen, sorbitol, or naltrexone), the additional agent will be considered irrelevant in case of FTO. And in case of landscape, the additional agent with these active agents will be considered relevant.
Structure-Based Analysis of Markush Structures
Now, let us consider the analysis of references based on Markush structures. Notably, Markush structures describe a compound class by generic notations like substitution, variation, frequency variation, position variation, homology variation. These structures describe smaller chemical libraries but in patents, they’re used to achieve broader technology coverage. Given below is one exemplary representation of Markush structure.
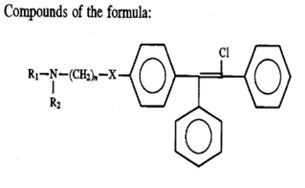
wherein R1 and R2 are each selected from the group consisting of C1-C2 lower alkyl groups; X is -NH or -S and n is a whole number from 1 to 4. When n is equal to 0, X is (CH2)3 and the pharmaceutically acceptable salts. These have been shown to be effective in treatment of tamoxifen-resistant cancers.
Conclusion: The Importance of Prior-Art Searches in Pharmaceuticals
Patent searches are vital in the pharmaceutical industry, not only to protect a company’s innovations but also to minimize the risk of patent infringement. Prior-art searches, including FTO and landscape analysis, help companies refine their IP strategy, optimize R&D investments, and navigate the complex patent landscape.
By conducting comprehensive keyword-based and structure-based analyses, pharmaceutical companies can effectively assess the risks and opportunities in the market. With the pharmaceutical industry becoming increasingly competitive, ensuring the freedom to operate and identifying the right technologies is essential for long-term success.
Ready to safeguard your innovations? Explore our Prior-Art Search services and take the first step toward securing your product’s future. o learn more about Prior-Art Search in the Pharmaceutical Domain, watch our webinar here.

-Harsha Agarwal (Life Sciences) and the Editorial Team
Having Queries? Contact Us Now!
"*" indicates required fields




