Performing Prior-Art Search in Pharmaceutical Domain – A Structured Approach – Webinar
Performing Prior-Art Search in Pharmaceutical Domain – A Structured Approach
Key Points to be Covered in the Webinar Session
- Differences in performing different type of prior-art search in Pharmaceutical Domain
- Considerations for performing prior-art search in Pharmaceutical Domain (Structure Search + Keyword Based Searching)
- Considerations for analysing patents
Table of Contents
Speakers
Ram Tenneti, EVP, Sagacious IP
Pooja Chhikara, Project Manager LSC, Sagacious IP
Harsha Agarwal , Project Manager LSC, Sagacious IP
Submit Your Information to watch the Webinar Video:
"*" indicates required fields
More details about this Webinar:
Prior-Art Search in Pharmaceutical domain involves challenges such as:
- Searching through the references disclosing the compound by its IUPAC Name, common names, salts of the compound of interest, compound’s exact structure, compound’s Markush Structure.
- The compound might be considered in its crystalline or enantiomeric forms.
- The role of inactive agents in the composition.
- Whether the formulation or the composition needs to be searched.
- Whether the route of administration/ or use is important for searching.
- A single Markush structure can evolve into millions of similar compounds; and therefore, the above pointers make searching in these domains more complicated and needs a structured approach.
Webinar Transcript:
Ram Tenneti Speaking – Good morning, good afternoon, good evening to one and all!
This is Ram Tenneti, the executive vice president at Sagacious, signing in from India. I welcome you all to our webinar today on the topic of “Performing Prior-Art Search in Pharmaceutical Domain – A Structured Approach.”
Now, before I go on to introduce this topic with our esteemed speakers on our session today, I hope you and your loved ones are doing well amidst this COVID crisis. Please be well and take very good care of yourself and everyone around you.
Thankfully, we have a very diverse spread audience in attendance today from all parts of the world: Japan, Switzerland, US, UK, France, Canada – I can see people from India, Mexico, Belgium, Italy, Poland, Ghana, Ukraine, Singapore, Australia, Bulgaria, China, Germany and Netherlands.
Well, we are very honoured to share our rich experience together, working with inventors, research and development organizations, IP departments, and IP Law practices.
The first speaker of the session is Harsha Agarwal. She is the project manager with life sciences and chemistry at Sagacious. Welcome to the webinar, Harsha.
Harsha Agarwal Speaking – Thanks, Ram – thanks for having me on the webinar!
Ram Tenneti Speaking – Great!
And our second speaker today is Pooja Chhikara. She’s the project manager with Life Sciences and Chemistry at Sagacious. I welcome her to the webinar as well.
Pooja Chhikara Speaking – Thank you, Ram – thank you for having me on this webinar!
Introduction to Prior-Art Search in Pharmaceutical Domain
Ram Tenneti Speaking – Wonderful!
Guys, before we start off with the presentation today, let me quickly ask our speakers for their initial remarks on today’s webinar topic “Performing Prior-Art Search in Pharmaceutical Domain – A Structured Approach.”
Harsha, would you like to start ahead with that?
Harsha Agarwal Speaking – Yeah, sure, definitely, Ram!
First of all, a very welcome to all the participants to the webinar! Today, we are gathered here to discuss prior-art search in pharmaceutical domain. When you talk about the pharmaceutical domain, this industry in itself is very unique than compared to its counterparts, and the main reason behind this uniqueness is the fact that high cost of research and development is involved in the development of one new single product.
When companies invest millions of dollars – if I have to give a number around $1.5 billion in the development of one single drug – when this drug eventually gets approved and is coming to the market, they expect to have certain exclusivity as to further products, so they want a patent exclusivity to market the product.
*
This leads to a scenario where most of the information in the pharmaceutical domain is present in the form of patents. Any information about the drug or its target or its efficacy is present in a patent, but patents are very techno-legal documents and thus going through the patent and extracting information through these patents can be very, very difficult or tricky. Thus, we need a structured approach to understand how to search in this domain, and, more importantly, how to analyse the references that we have discovered in the searching. So, that is overall why I think talking about the structured approach for patent searching is very important in this particular domain.
Thanks Ram!
*
Ram Tenneti Speaking – Thank you, Harsha! That is very interesting insight and we hope that you will be shedding some more information on that. And why don’t we ask Pooja to share her initial remarks?
Pooja Chhikara Speaking – Yes, I guess, Ram!
Well, a pharma domain, indeed, includes a research of several years unlike other domains. It includes complex chemical names and structures which are difficult to understand and require specialised databases, and when we talk about MARKUSH structures specifically, a MARKUSH structure may result in millions of possible structures which make searching in pharma domain a tedious task.
So, in this webinar, our focus would be to simplify and elaborate step by step on things that are important for consideration in patent search and analysis. So, that’s what my stand is. Thank you, Ram!
Ram Tenneti Speaking – Thank you Pooja, and thanks Harsha and Pooja for setting the context for the webinar. It sounds really interesting. Before we move ahead, I would like to invite all our listeners. All the participants can drop us a line, or an e-mail later: webinar@sagaciousresearch.
Prior-Art Search in Pharmaceutical Domain and Its Challenges
Let us now get started with the topic of our presentation, and for that, let me quickly invite Harsha to take us through the webinar. Over to you Harsha!
Harsha Agarwal Speaking – Thanks Ram!
So, before I start with this presentation, I would like to take this opportunity to quickly introduce myself. My name is Harsha, and I work as a Project Manager in the Life Sciences domain of Sagacious IP, and here, at Sagacious IP, in the Life Sciences domain, we perform prior-art search in the form of FTO, landscapes, novelty, invalidity searches, and also market research in the specific pharmaceutical and other aspects of the life sciences domain.
As we discussed in the initial remarks, that pharmaceutical domain or the industry has its own set of challenges. Before we deep dive into the “Hows” and “Whys” of searching in this particular domain, we would go through – what makes this domain or industry unique and what are its challenges.
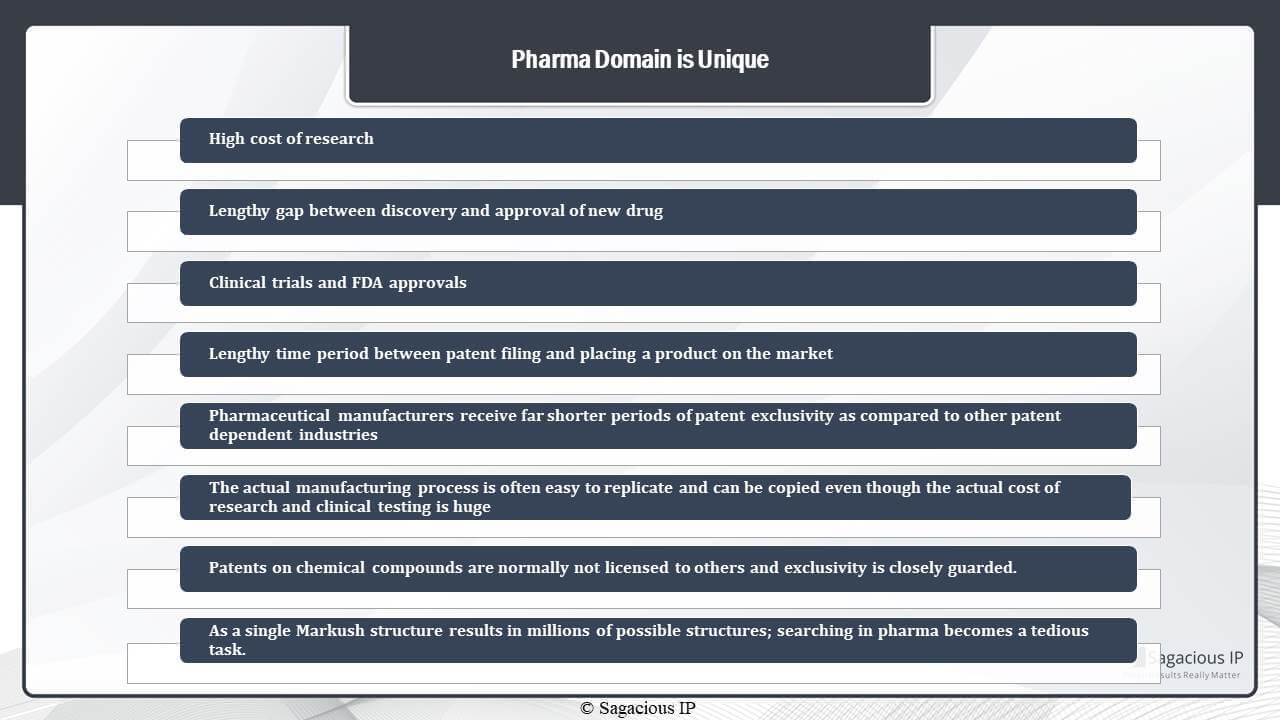
As we discuss, the main reason behind the industry being unique is the high cost of research. Because years and years of research go into the development of a new drug, there is a lengthy gap between the discovery and the time variable to get it marketed. Also, because a pharmaceutical domain deals with products that are directly consumed by humans, there are very stringent regulations around the products and thus the approval of a new drug can be very lengthy and tedious. Clinical trials are usually performed both on animals and humans before a drug is allowed to be marketed. This also leads to very lengthy gaps between the marketing of the new drug.
*
What happens is when a product is discovered and finalise a patent is filed, but even when a patent is filed, it cannot go to the market before the approvals, and thus there is also a lengthy time period between the patent filing and the placing of the product in the market. Thus, what happens is that the pharmaceutical manufacturers or companies – they get far shorter periods of patent exclusivity as compared to other patent dependent industries, and this all happens because of the lengthy time period. Thus, these pharmaceutical manufacturers expect exclusivity on their products. Another reason for this is that the actual manufacturing process of the drug is usually easy to replicate and not that complicated. The actual cost and time go into the research part and clinical testing part.
Again, when we talk about patents in this domain, these are not usually license to other people or companies in the same domain, and this is the reason because the patent companies want to enjoy exclusivity in the market so that they can get a return on their investments. What makes prior-art search in the domain unique is not just the fact that a lot of information is present in the patents. It is also because the information is present in a very complicated form.
For example, if we talk about MARKUSH structures which are broad chemical structures that can cover millions of possible structures within one single backbone so that searching in this industry becomes more tedious and tricky.
When You Should Request Prior-Art Search?
Now, when we talk about prior-art search, one can ask when to request or conduct a prior-art search, and how will it help them in the process of R&D.

The first and foremost stage, at which a prior-art search can be requested, is before planning R&D. This is done in the form of a landscape to assess the work that has been already done, and, also, to ensure that the work that the company is going to do is not a duplication of already existing research.
The second stage of conducting a prior-art search in pharma domain can be the stage where the patent is going to be filed. This is usually conducted to identify closely related literature, and it’s also highly recommend so that any literature that can be a prior-art to your patent is identified. This will help you in devising the answers to the examiner’s questions and rejections and also help you work around the existing prior-art. All in all, conducting a prior-art search in pharmaceutical domain before filing a patent saves a lot of time and money.
The third stage, where a prior-art search in pharma domain can be requested, is just before the product launch. After a product is completed and it has to go to the market, a freedom to operate search is usually conducted to identify any patents that this product could be infringing.
Now, after the product is in the market, there can be a stage where your product is already infringing a patent that you were not aware of. So, in this case, opposition is an option where the patent that is being infringed on can be opposed by searching prior-arts in the domain that could invalidate the claims of novelty or obviousness for that particular patent.
FTO Searches in Pharmaceuticals
If we talk about freedom to operate searches specifically in the domain, then, again, it could be conducted at three stages. It can be conducted before the approval of a research project, but the most recommended stage to perform these searches is after the completion of development and before marketing, because, at this stage, the search can be precise and can be used to identify the very most relevant references.
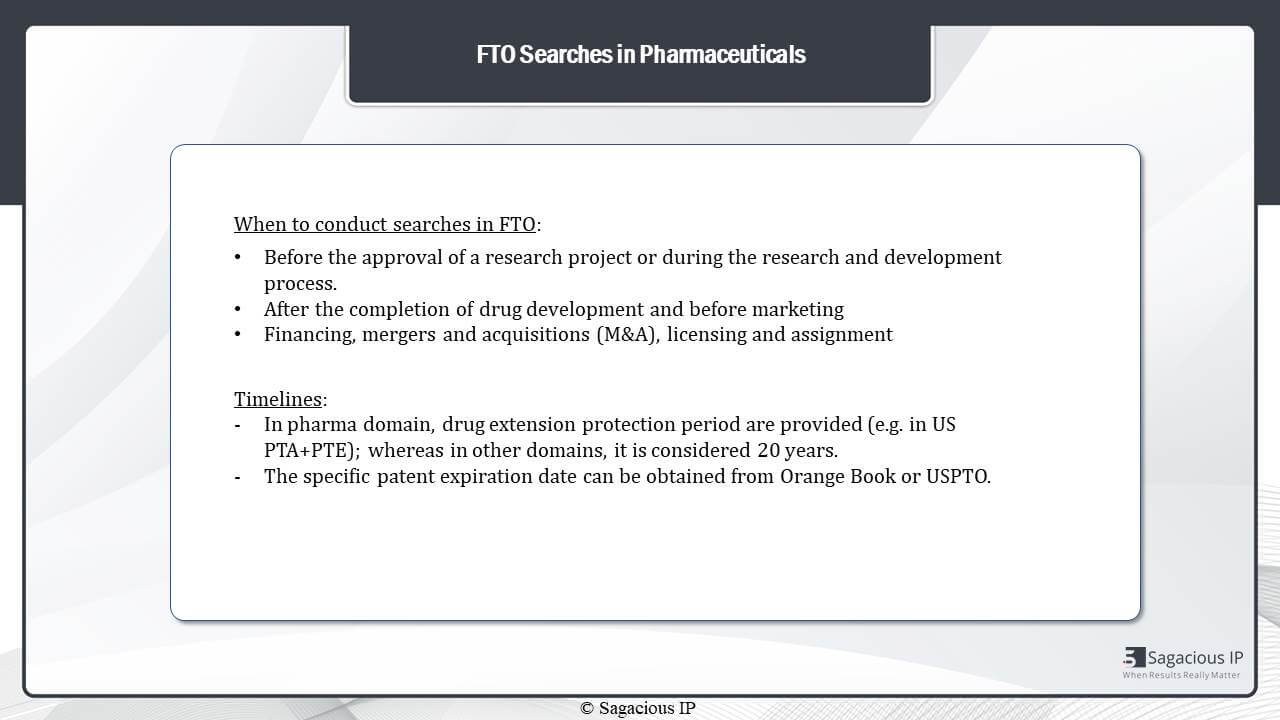
During mergers and acquisition or licensing agreements or decisions also FTO can be performed to assess the invention in question. In FTO searches in this domain, timelines are a bit tricky as compared to other domains because, sometimes, patents for the drugs get extension protection periods. In U.S., It is provided as PTE while as in Europe it can be provided as SPC.
An overall timeline for one patent in this domain can be more than 20 years, and this information can be obtained from Orange Book or the USPTO. These are very important while designing an FTO search in this domain.
Prior-Art Search in Pharmaceutical Domain: Considerations & Best Practices (Keyword Based)
Now we’ll talk about overall considerations and the best practices – the do’s and don’ts of performing prior-art search. Initially, I would focus on the keyboard based searches that we performed in the domain.
Part 1: Term Sets Preparation
The foremost part or step of performing a keyword based search is the preparation of terms. So, once a technology is in hand, we extract keywords or concepts that are important for the technology, and using those keywords, we prepare term-sets by the synonyms of those keywords to identify a maximum patent literature, and in case of a chemical formulation or a pharmaceutical formulation composition four different aspects can be thought of.
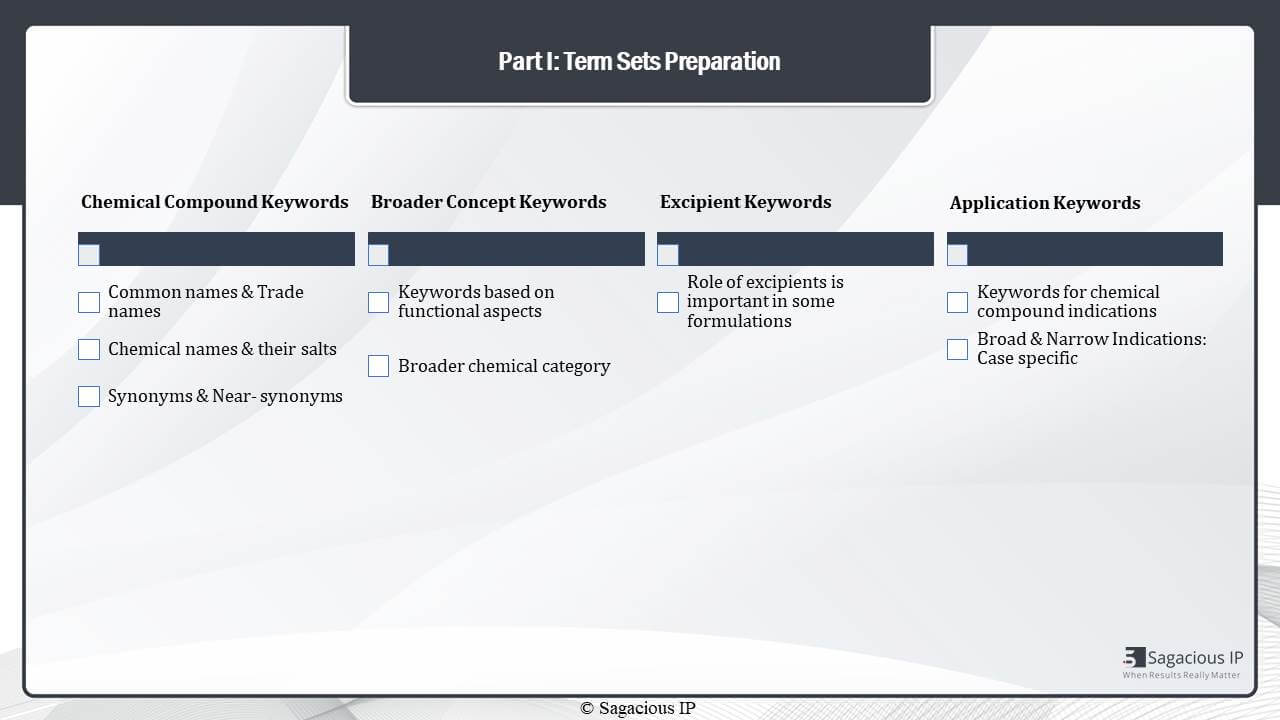
- The first is the active ingredient or the agent itself which is the chemical compound or whatever the active agent is.
- The second is the broader aspect of the active agent.
- Then, there can be excipients or other adjuvants in the formulation itself that may or may not be playing a very important role.
- The fourth aspect is the application which is talking about the indications that formulation may be used for. So, for each of these type of keywords, we use different types of synonyms, and we make sure that the term-sets that we prepare is very exhaustive.
*
Now, let us go through all of these aspects in the form of an example. So, the case study that I have taken here is Ribociclib formulation which is an anti-cancer agent. So, in case of the formulation – Ribociclib formulation, if I were to prepare term-sets for it, I would start with the chemical compound itself which is Ribociclib. Of course, the Ribociclib term is included, but I would make sure that I include the trade name, other names by which it might have gone into clinical trials – of course its IUPAC name and CAS number as these are unique to every chemical compound – also, variations or synonyms or near synonyms of the IUPAC name of the compound. These information is usually are extracted from resources like PUBChem or ChemSpider.

The second aspect is the broader keywords.
So, once I am done with the Chemical compound term-sets, I would like to see whether there can be patents that would disclose the information in form of a kinase inhibitor since Ribociclib targets Cyclin D1 or CDK4. So, I would ensure that I use term-sets that talk about inhibition of cyclin or kinase inhibitors in general.
Another way to create broader chemical names is to use the broader chemical category or the backbone of the chemical compound. In this case, Pyrrolopyrimidine is the backbone or the chain of Ribociclib, and thus I’d include such terms to ensure that Pyrrolopyrimidine derivative is also captured in my prior-art search.
The third aspect is the application or use related
It is because Ribociclib is used against breast cancer. I would include terms for breast cancer. I can also make these terms for indications broader where I would take cancer, tumour, et cetera, to make sure that all indications are captured. Lastly, other components, excipients, in the formulation, if they are playing an important role, can be used as keywords and terms that are prepared.
In this example, if it’s a micro particle, I would use terms like micro particles, and also the polymers that are being used to create the micro particles as a part of the term-sets. So, I hope we’ve covered, here, the major aspects of preparing term-sets.
Part 2: Class Based Searches
When we move forward, there is also another prior-art search in pharmaceutical domain strategy that is employed which is to use classification based searches for identifying references. The first step in class based searches is to identify the IPC or CPC. This can again be identified based on different aspects which are similar to keywords, the functional groups, the target of chemical compound, the indications, or excipients.
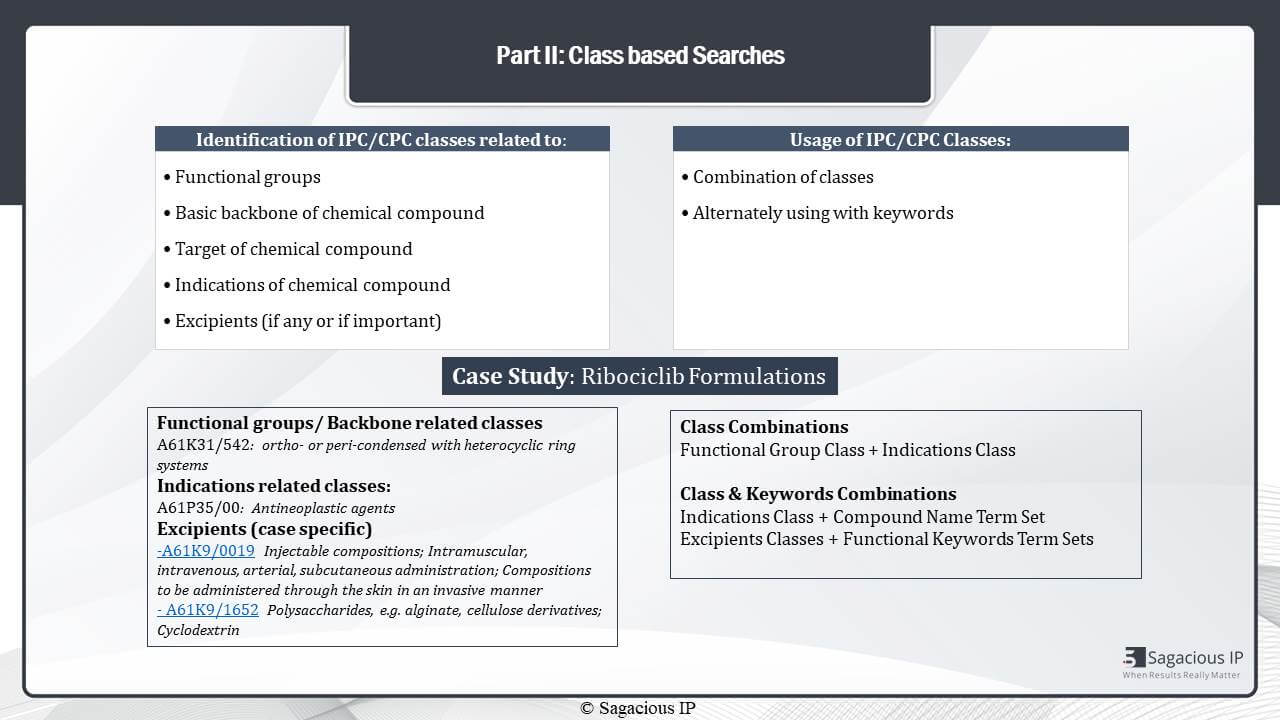
So, as we see in the same example – the Ribociclib example – when I talk about the functional group or the backbone, we have identified a class which talks about the heterocycle ring, then, because this is an anti-cancer agent, we have also identified classes related to its antineoplastic properties, and since this composition was in an injectable form, the type of dosage form is also included, and, again, excipients are important, so excipients were also included.
Once we have identified the important classes, then we can use them in various ways by combining them with keyboards, or even combining two different classes. It is always preferable, though, to use a set of keywords and a set of classes which are of two different aspects. So an overall holistic approach can be used to search these patents.
In this case, for example, if I have taken the class for indication, then I have used the term-sets for the compound name. Similarly, various strategies can be formulated by playing around with the term-sets and the IPC or CPC classes.
Part 3: Additional Searches
Additionally, with the mainstream keywords and class based search that we do, there are also searches like assignee or inventor based searches that are employed in most of the domains for doing a prior-art search in pharmaceutical domain where assignees are the prolific in the domain or specifically for pharmaceutical, we can take research groups or labs that are working on specific indications.
For example, if we talk about cancer, there are so many groups that are working on similar targets of the cancer, and then we can identify those groups and see if those assignees have patents and similarly for inventors, we can identify inventors and perform such searches.
Citations searches have always been an integral part of prior-art search in pharmaceutical domain.
Source
Here, I have listed some of my sources that we use, at Sagacious, here, for keywords, for classes, for assignees, and other drug related information. We’ve already talked about most of them. Research papers that are related to the domain are another very important resource that has to be used at every state while developing term-sets and, also, while analysing patents to understand how patents are or information have been disclosed.

So, this was an overview of how we conduct keyword and class based searches.
Prior-Art Search in Pharmaceutical Domain: Considerations & Best Practices (Structure Based)
Now, because we’re specifically in the pharmaceutical industry, that is why the chemical structure or a chemical formula becomes very important, and thus searching prior-art based on the structure of a compound becomes more important here.
Part 4: Structure Based Searches
Structure based searches are usually conducted on databases like STN where the target compound structure is used as a query to identify other patents that would be disclosing similar chemical structures, and thus, in that way, we’ll be able to identify such patents.
Usually, the structure based searches can be of three types broadly.

The first one is the exact structure search. This one, as the name suggests, is very simple. You give the query compound, and it’ll return references with the exact same structure. The reason or the negative aspect of this exact structure search is that it will not capture salts or polymers, but it is very useful in performing novelty or invalidity searches.
Substructure search is the one that is used the most because it looks for the query structure as a part of a bigger compound also. Thus, it returns references are compounds with its salts and hydrates and also identifies polymers if the monomer is indexed.
Similarity search, on the other hand, goes on to the other extreme of becoming too broad where they identify structures with a similar motif, and thus multiple references can be identified. This can be used in cases where a very, very broad scope of the search is required.
Now, that we’ve covered the types of searches, we will talk about the basic method of performing this search, and so the main three steps of performing these searches would be, first of all, to draw a query or chemical structure on suitable software like ChemDraw, then, after we’ve done that, the substituents are used to ensure the scope of the search.
Example
For example, in any compound, if there is a substituent R, the two extremes of the scope would be Methyl on one end of the spectrum where it would be very narrow and could only capture Methyl at the substitution point, and the other end would be hydrocarbon which would identify any hydrocarbon at that position.
Once we have performed a search, we need to optimize the search. This can be done by scanning the sample search that STN lets us perform, and once we have scanned through the references, we can identify what kind of references are being captured, and if the number of hits is too high, then we can restrict specific substituents to ensure that we’re covering the scope that we are looking for.
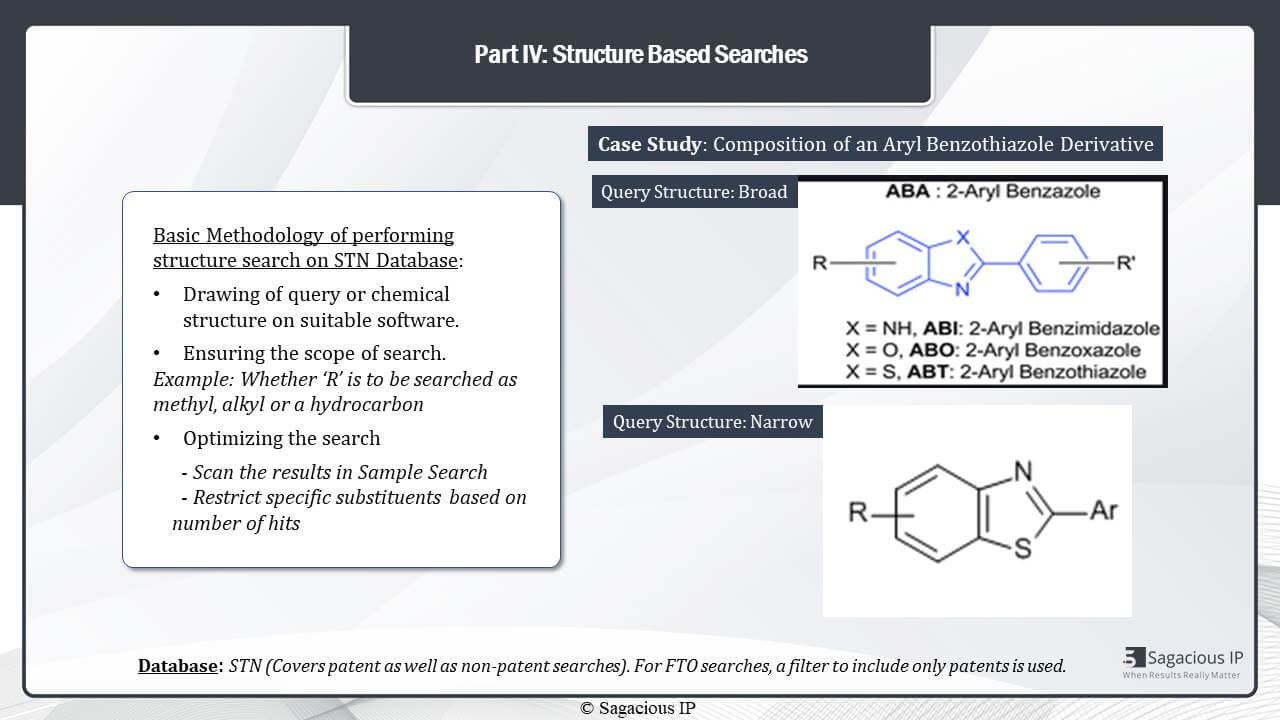
*
Let us just see this optimization and scope with the help of an example. In this case, study, the composition was of an Aryl Benzothiazole. So, the first type of query structure that could be here is broad where I have left the X open. Now, the X can be anything from NH, O to S, and depending on what X is, the compound will change. We only need X to be S in case of a Benzothiazole.
In this case, it would be preferable to search if you’re looking for the specific compound only and its derivatives only. We will use a narrow structure, query structure, where the X is not left open, and it has been restricted as an S only so that all the references that are captured through the query structure are Benzothiazole derivatives.
So, and, again, like other such substituents can be left open or be restricted based on the scope of the search similarly.
STN is a database that lets us cover both patent and non-patent literature. During FTO searches, we use a filter to include only patents, but during novelty and invalidity searches, both patent and non-patent literature can be captured using one single query structure and one search.
Overall, this was the overview of performing both keyboard class and structure based searches in the pharmaceutical domain, and now my colleague Pooja Chhikara will take you through how to analyse and identify relevant results in this domain.
Over to you Pooja!
Analysis & Identification of Relevant Results ( Keyword Based)
Ram Tenneti Speaking – Well, thank you Harsha for the comprehensive overview of prior-art search in the pharmaceutical domain. Why don’t we ask Pooja to take us to the analysis section.
Pooja Chhikara Speaking – Thank you, Ram, and thank you Harsha for a well elaborated explanation of important aspects of searching in Pharma domain! Moving forward, now, I will discuss the key aspects in the analysis of pharma patents. First, let’s start with keyword based analysis.
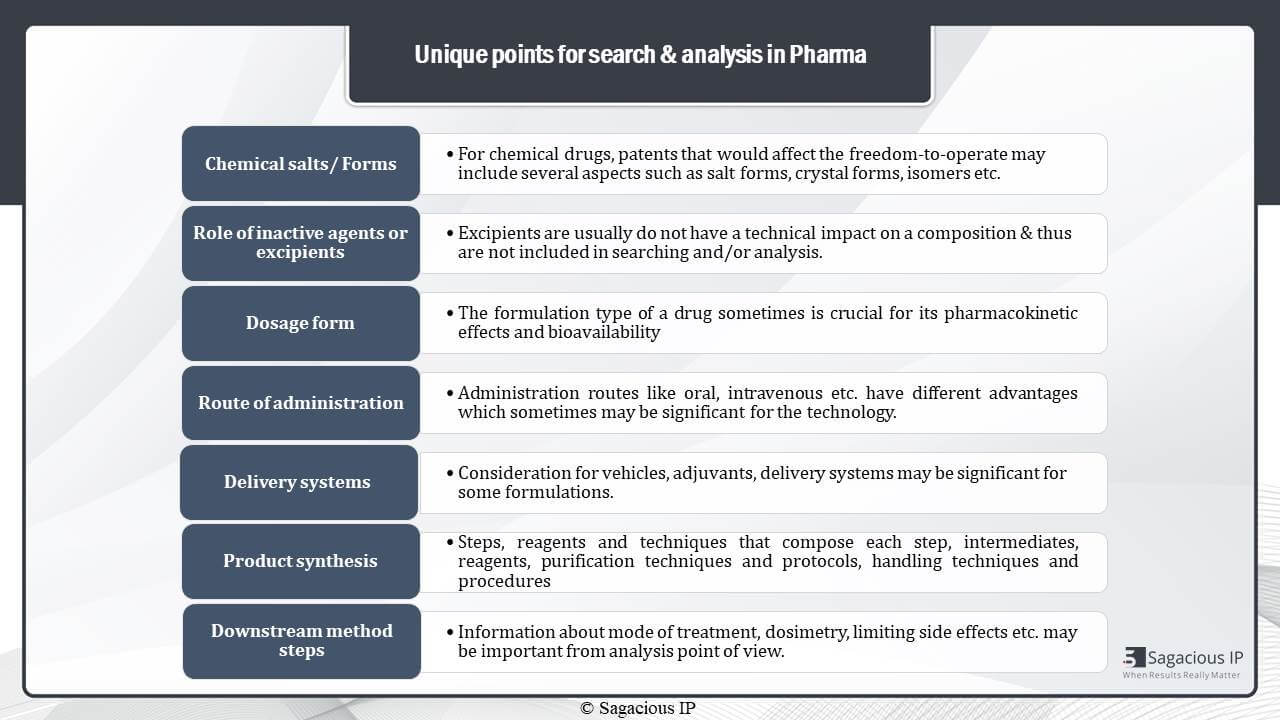
When we talk about pharma domain or drugs or chemical agents, the unique points for patent analysis in this domain are:
Number one is chemical salts as a drug has various forms of salts, isomers, or crystals.
Number two is Excipients. Generally, excipients do not have technical impact on a composition, and thus they are not included in searching and analysis, but in some cases, they may impact the efficacy of the formulation and need to be considered there.
The third is dosage form, and number four is route of administration. A drug can be present as a pill, syrup, powder, liquid, aerosol, inhaler, etcetera, and it can be administered orally, nasally, intravenously, or via ophthalmic means, et cetera. Hence, these two parameters are considered important for analysis when they are crucial for pharmacokinetic effects and bio-availability of a drug.
Number five is delivery system. Delivery vehicles and Adjuvants – they need to be considered during analysis when they are important in drug formulations.
Then, number six is product synthesis.
In a drug synthesis technology, the methodologies steps, reagents and techniques that compose each step, intermediates and handling techniques are crucial for analysis considerations.
The number seven is downstream method steps like information about dosimetry, limiting side effects, et cetera. They may be important from analysis point of view.
Chemical Salts/Isomers/Crystals
Now, let’s discuss some of the above mentioned parameters in detail.
The first is chemical salts. Salt forms of drugs are important as they have significant effects on physiochemical properties of the drug, influencing its quality, safety and performance. Importantly, these salt forms really change a drug’s pharmacology property.
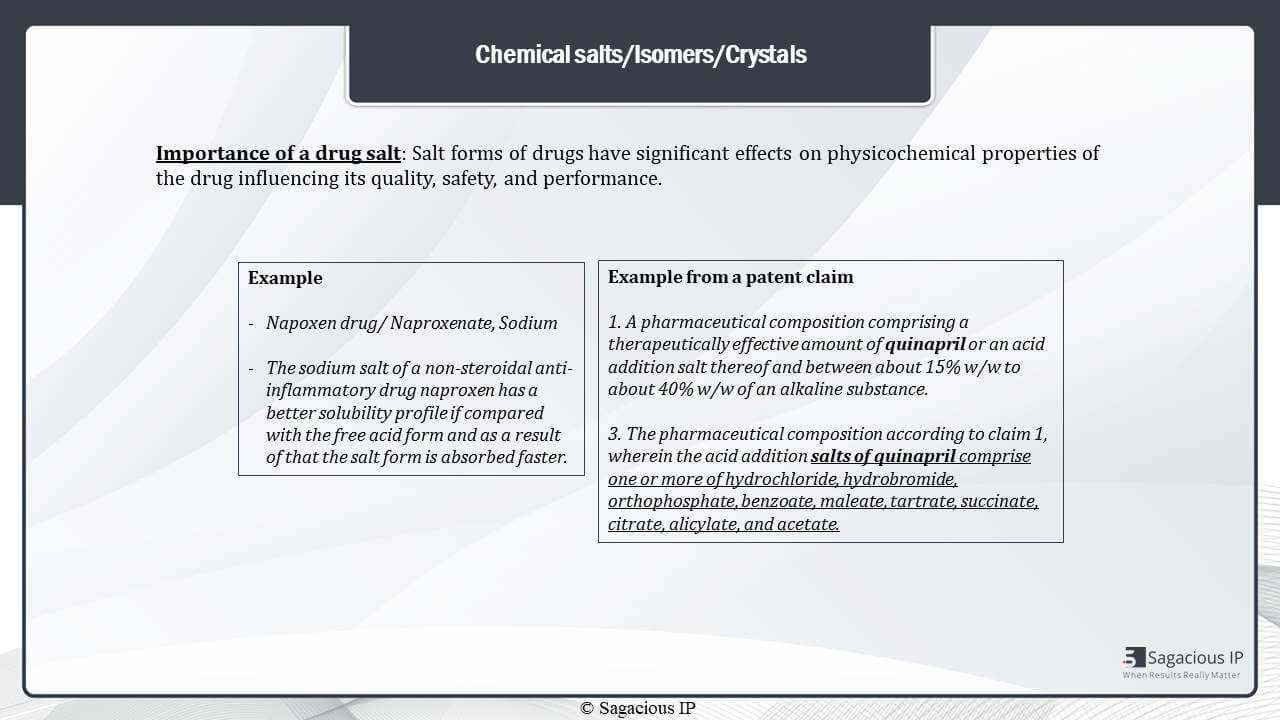
For example, salt of Napoxen drug is Naproxenate Sodium. The sodium salt of the drug has a better solubility profile when it is compared with the free acid form and as a result, this salt form is absorbed faster.
Now, here in the claim example, it is shown how different salt forms of quinapril are protected by the applicant. Hence, all salt forms of a drug are important to take into consideration for search and analysis.
Excipients
Further, excipients are considered important when they are being used as active substances.
For example, when glycerol is acting as a preservative or Ascorbic acid is acting as anti-oxidant. The same is shown in this example, here, wherein a pharmaceutical composition comprises Prolactin and Ascorbic acid to effectively modify the amount of type 3 collagen in connective tissue.
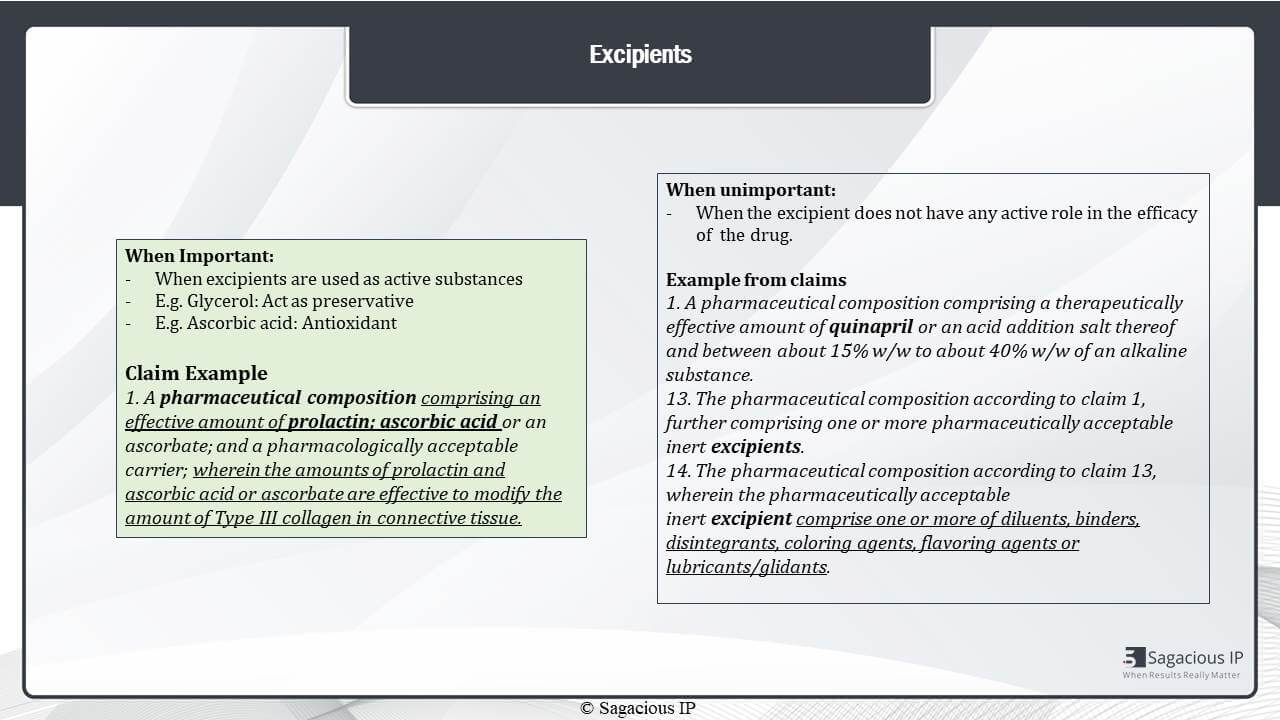
Then, the excipients are considered unimportant when the excipient does not have any active role in the efficacy of the drug. This example here shows a pharmaceutical composition of Quinapril along with a regular excipient with no active role.
Dosage Form & Route of Administration
Next, this dosage form and route of administration – they are meant important when this stability and efficacy depends upon them.
For example, in one of the technologies, solid dosage form of insulin is claimed. Here, the dosage form is important as insulin is generally injected in body, because the compound is not stable and effective enough to be ingested orally.
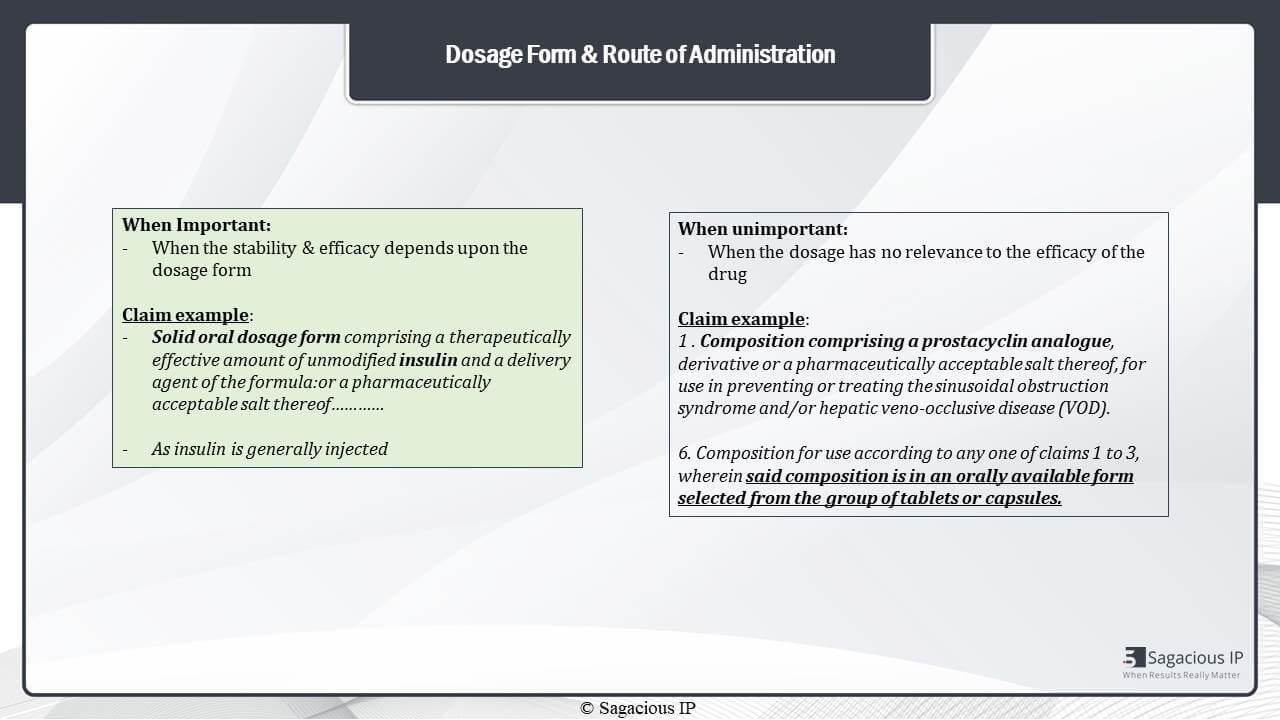
If we see on the right side, the dosage form has no relation to the efficacy of the drug. It is considered unimportant. The example shows a regular dosage form of Prostacyclin analogue.
Delivery System/Adjuvants
We have delivery system or adjuvants. First is Delivery Vehicle. Herein, an example of technology, is shown, which is majorly focusing on drug delivery system comprising polymeric hyaluronic acid vehicle associated with the antibody Bevacizumab.
In cases like this, they are important for search and analysis as the focus of the technology is on the delivery vehicle. Then, talking about adjuvants, they are generally immunostimulants, for example, LPS, saponin, et cetera and used mainly in vaccines.
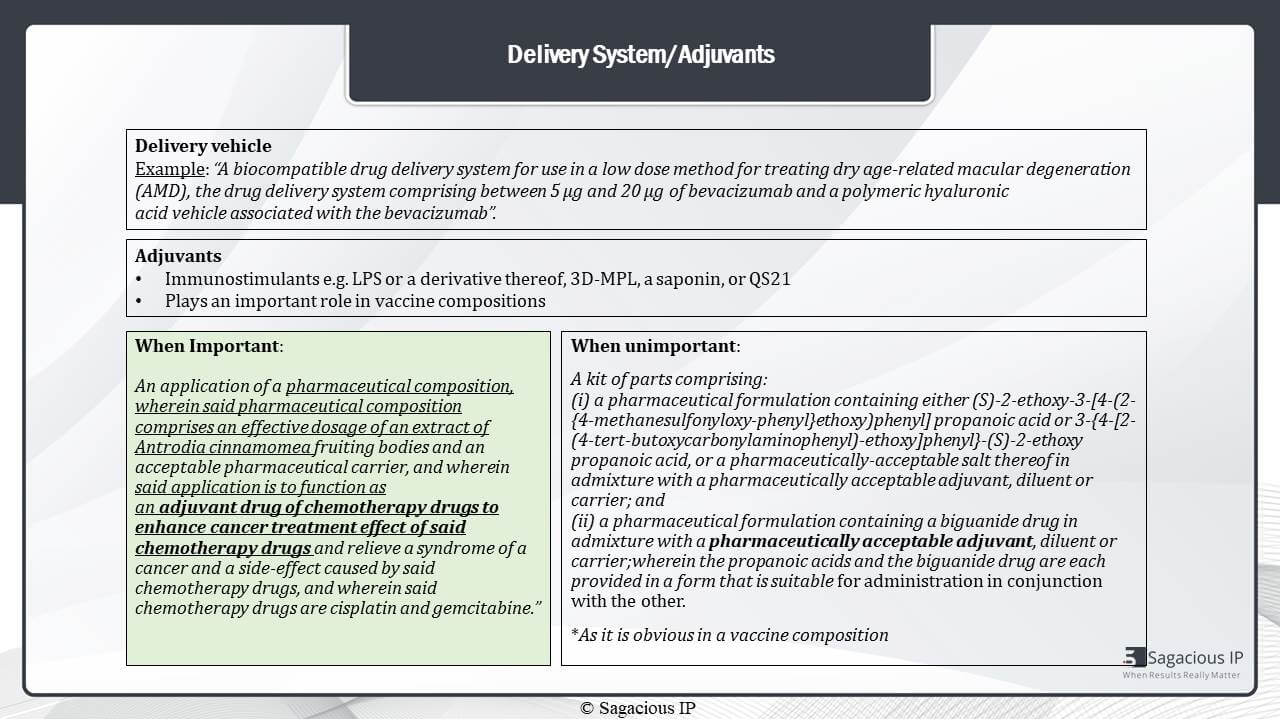
The adjuvants are considered important when they are playing an important role to enhance the treatment by drug as shown in this example here, and they are considered unimportant when they are added as a regular additive in a drug formulation.
Evaluating Relevance of Results
Till now, we were talking about the common agents of pharmaceutical compositions and methods of synthesis. Now, we will throw some light over how the relevance of patent is derived, starting with FTO or the freedom to operate search.
Like suppose, there is an invention claiming a formulation comprising A+B+C+D, then any patent claiming A+B+C would be considered relevant, and the excipients present in formulations are not taken into consideration while evaluating the relevance.

Third, claims covering only 1 or 2 of the components of the invention formulation are not a direct threat to the technology, and thus, they are not considered as relevant but as potentially relevant and they can be further analysed to assess the risk.
Then, fourth is: claims having additional components in combination to the pharmaceutical drug formulation are not considered relevant in case of FTO, but talking about landscapes, combination with additional active agents or having other applications may be considered relevant as the scope of landscape is wider.
Then fifth, a broad claim covering the concept of the invention but not specific aspects of the invention is not generally relevant but may be considered as potentially relevant.
Examples
For example, in case of search for Ribociclib, references disclosing kinase inhibitors broadly may not be considered relevant but potentially relevant. This was in the case of FTO. Now if we talk about this same case for landscape, then very broad claims are usually considered non relevant.
Further, independent claims having a specific use or indication where we don’t know the use of drug of interest, they’re generally not considered relevant for formulation FTOs and may be considered as similar or related patents.
Additionally, other aspects like route of administration, dosage form, etcetera, are case specific, and they may or may not impact the relevance of a reference. We have already covered the differentiating points between FTO and landscape above.
Example for Evaluating Relevance
Now, let us understand the above mentioned points through these examples.
The example one can be considered equivalent to our composition A+B+C+D wherein A, B and C are baclofen, sorbitol and naltrexone, and D is excipient.
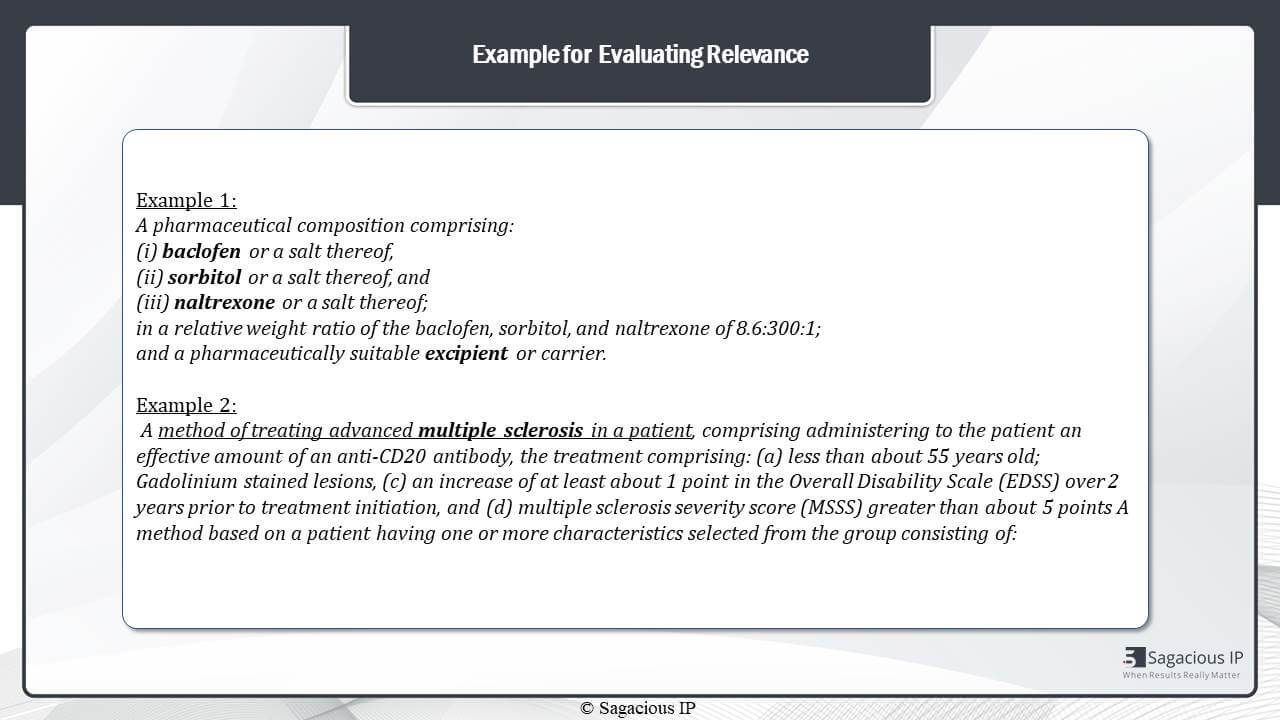
In the analysis point of view,
the first point is any patent claiming baclofen, sorbitol and naltrexone is relevant. And second, the excipient is not fit for analysis as it is a regular additive.
Third, patents claiming only baclofen, sorbitol or naltrexone is potentially relevant. As they do not pose a direct threat to the technology.
The fourth point is patents claiming additional agents in addition to these three active agents is non relevant in case of FTO. And in case of landscape, the additional agent with these active agents is relevant as we discussed in the previous slide.
Further, to elaborate the point of analysing an independent claim having a specific indication i.e., multiple sclerosis here. So, for an FTO formulation, such patents will be marked related or similar, but not relevant wherein the use or indication of drug formulation of our interest is unknown.
Analysis & Identification of Relevant Results ( Structure Based)
That was about the keyword based analysis. Now, I will talk about analysis of references based on MARKUSH structures.
What is a MARKUSH structure?
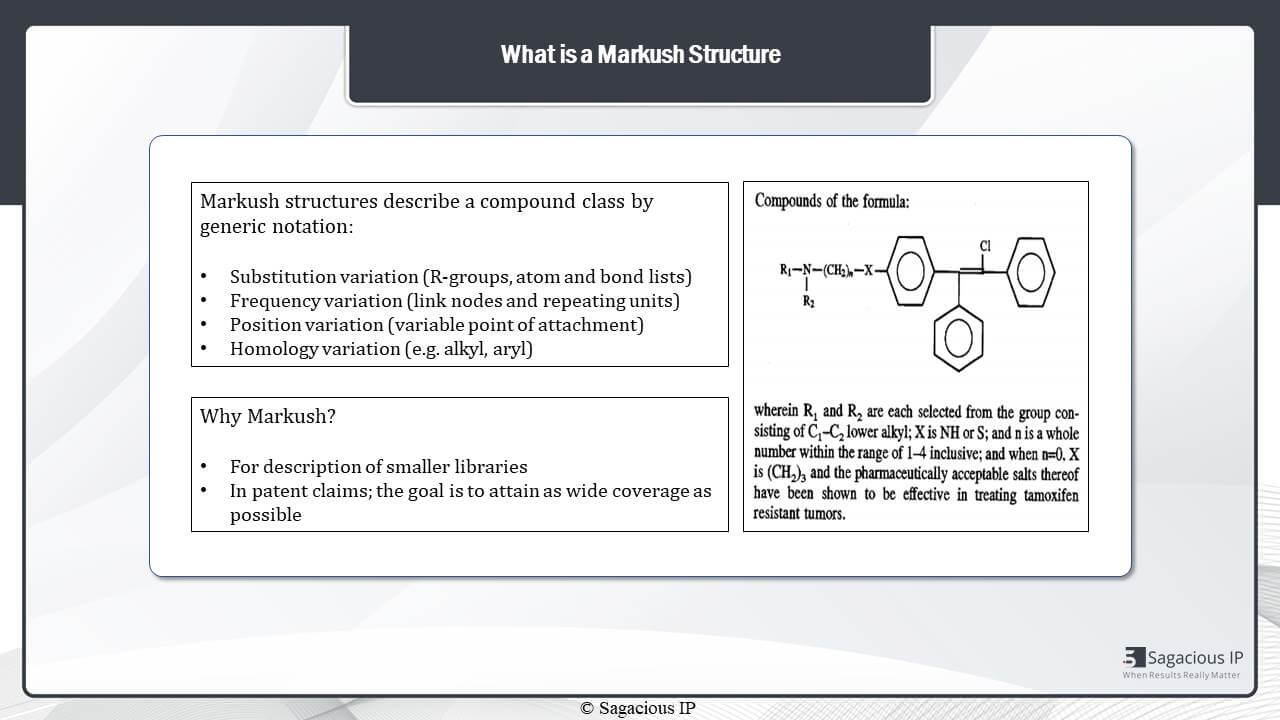
A MARKUSH structure describes a compound class by generic notations like substitution variation, frequency variation, position variation, homology variation, and MARKUSH structures – they are generally used to describe the smaller libraries of similar chemical structures; whereas, in patents, they are specifically used to attain wider coverage of the technology. Then, we have shown one exemplary representation of MARKUSH structure wherein R1 and R2 groups are shown broad, then they are narrowed down in the text below.
Evaluating Markush Structure Based Results
Now, I will elaborate on how the MARKUSH structures are analysed. Like, here, we have taken a case study of Ribociclib again. This is the query structure of Ribociclib. Now, let us see how a relevant reference would look like.
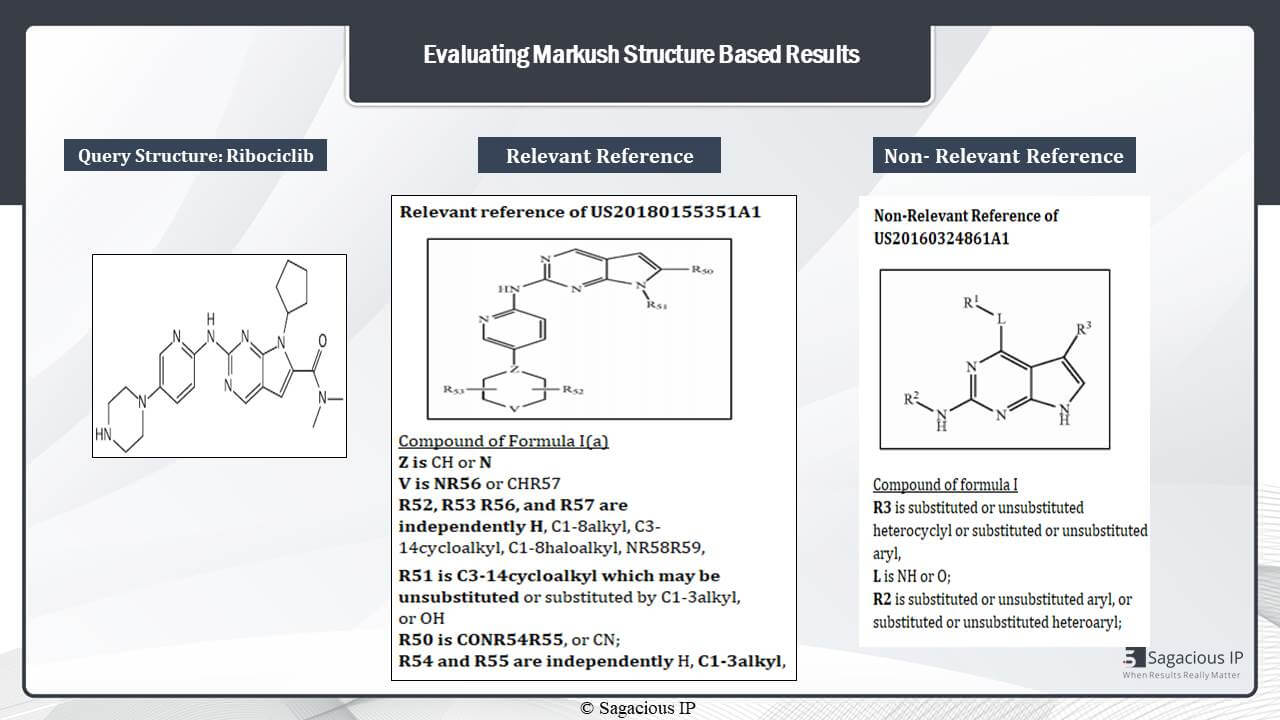
If we compare the structure of this reference with the query structure, then we can see that these three things are exactly same with the linkage of NH between them. Then, moving to this ring, we can see that, here, we have Z in place N, then V in place of NH. We have to check this Z. Z is N, then this V is NR56. Now, we have to check this R56. R56 is H, and if we see these R52 and R53 groups, they can also be H. So this ring is okay.
Now let’s look for this substituent. This is R51. So, R51 is C3-14cycloalkyl which may be unsubstituted, and this satisfies this ring which is a five member cycloalkyl ring. Now, let’s look for this group. This is R50. Now, R50 is C0 that is C double bond O, and then N, then we have N here. Now, generally these two bonds – they depict the alkyl groups. So, we have R54 and R55. These two groups are C1-3 alkyl. So, this whole structure is okay, and it is relevant for us.
Let’s move to see what a non-relevant structure would look like.
Yes, in the non-relevant reference, any difference in these substituents makes a structure non-relevant. So, if we look at these structures, we need to have this cycloalkyl ring at the end position, but we don’t have any ring at this position. So this is a differentiating factor. Then, we need to have a substituent at the second position from N, but we don’t have any substituent here. Instead, we have an additional substituent R3 at this positon and this R1 linkage here which is also an undesired substituent. So this is a non-relevant reference for us.
Well, that was all in the analysis of Pharma patents. Now, my colleague Harsha will give you a summary or recap of the whole session.
Summary
Harsha Agarwal Speaking – Yes, thank you, Pooja! That was very elaborative and in-detailed explanation of how to perform analysis in the domain. So, just to summarise what all we’ve covered today through these presentations, I will take you through just an overview of prior-art search in pharmaceutical domain.
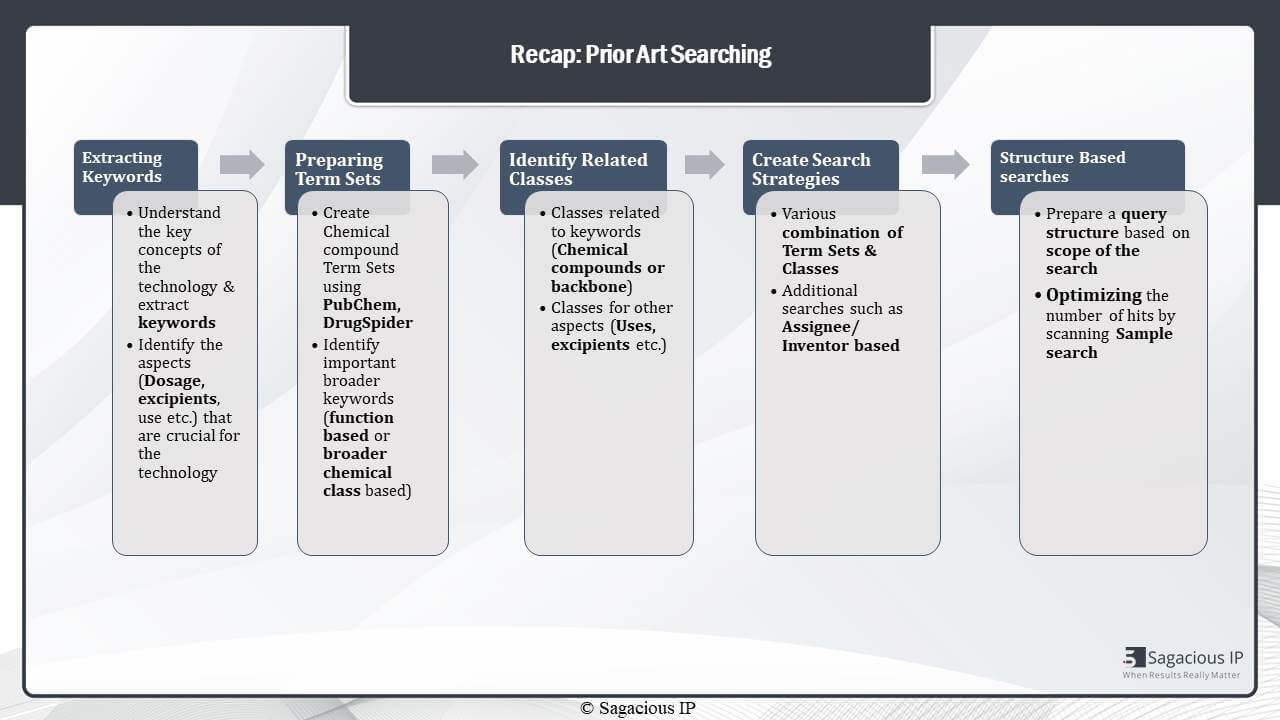
Prior-Art Search
The overall prior-art search in pharmaceutical domain can be divided into these five steps. Where the first and foremost important thing to do is to extract important concepts and keywords from the technology. In this case, we also have to understand which aspects are important. Whether dosage is important or not, and whether excipient is important or not. Similarly, determine all those important keywords.
Once we know what keywords are, then we create the term-sets using various sources. Like PubChem, ChemSpider, and other research articles related to the domain. At the same time, we also identify broader keywords that could be function based or broader chemical class based.
The third step is to identify the related classes. Again, these are based on different aspects of the technology. For example, the indication, the other excipients of the formulation. And, of course, the main chemical compound of the formulation.
The fourth step, which takes everything that we’ve done so far together. It brings everything done together, is creating prior-art search and pharmaceutical domain strategies. Where various combinations of term-sets and classes are used. Again, we can also perform additional searches such as assignee or inventor based or, also, citation searches.
An additional part in pharmaceutical searching is also structure based searching. Where MARKUSH structure can be identified or any particular chemical structure can be identified. It is done using a query structure on databases like STN. And, here, also, it’s very important to ensure that the scope of the search is very well-defined.
Analysis & Evaluating Relevance
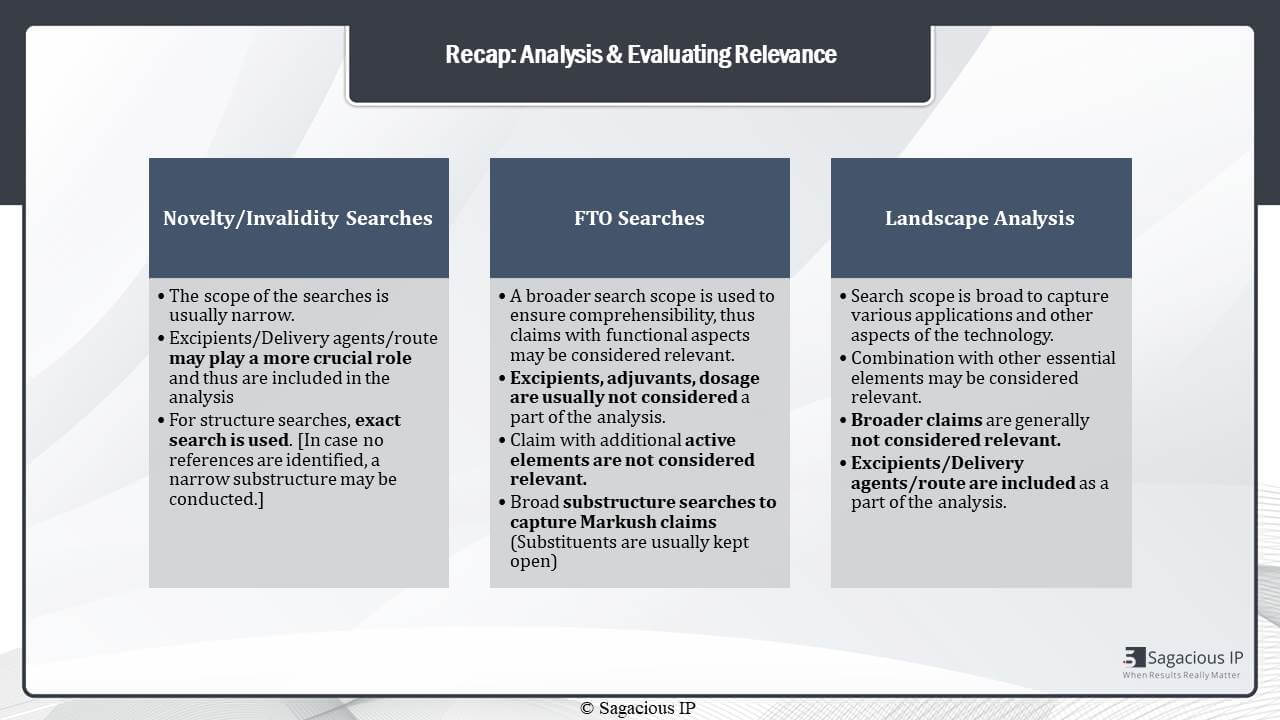
That was an overview of how to perform prior-art search in pharmaceutical domain. Once we have our references from the searches, we want to analyse and evaluate. The analysis part differs as per the type of search. If we talk about novelty and invalidity searches. The scope of the searches is usually very narrow, and thus, in this case, every aspect is important. Like excipients, delivery agents or even the dosimetry, offered technology could play a very important role in the analysis part.
Similarly, for structure based searches, we usually use exact structure search. Like the exact structure that we want to identify for the novelty aspect is captured. In case no references are identified, we may use a narrow version of the substructure search.
Then, in FTO searches, the search scope is usually broader. It is done so that to ensure that everything is covered. We do not miss out patents that could pose as a risk or a threat later. And in this case, thus, during analysis, excipients and adjuvants may not be very, very important. If the compound or the formulation, in general, is disclosed, it is considered as relevant. But, at the same time, if the claim has an additional active element, then it is not considered relevant.
In talking about structure searches,
Usually broad substructure are used to capture broad MARKUSH claims. And substituents are usually kept open, because if we are looking for a substituent say Methyl. Than even an Alkyl would cover the scope, and thus such structures and substituents become very important.
Similar to the FTO Search, there are landscape searches which have wider scope, but the wider scope is in terms of the applications or different aspects of the technology and not exactly in the broad scope of the claim. That is why, in this case, patents with combinations may be relevant. Even other uses and indications can be considered relevant. Very, very broad claims, which only talk about the general concept of the technology in hand. But do not specifically focus on that technology, are not usually considered relevant.
In this, again, excipients and delivery agents become important. It is because we want to see what all is happening in the domain. And we want to see a larger picture, and thus different aspects of the technology becomes important.
So, that was from our side talking about prior-art search in pharmaceutical domain.
Thank you all for your attention, and hope you have a good day.
Over to Ram!
Questions
How to Cover Every Name of a Chemical Compound While Preparing the Term-set?
Ram Tenneti Speaking –
Thank you! Thank you for this elaborate coverage, both of you Girls, Pooja and Harsha. I’m sure the audience must be having a lot of questions. Well, you have got me thinking – a quick question probably to Harsha. While preparing the term-sets for a chemical compound, how do you ensure that multiple variations of chemical names are covered?
Harsha Agarwal Speaking –
Sure, Ram! So, we have to talk about how we ensure that multiple variations of the chemical names are covered. I think this can be done in three ways majorly. Where the first way would be to ensure that you are using multiple resources and not depending on only one. It is because there may be some synonyms of the IUPAC name. Or the chemical name that are present on one resource. And are not present on another because there are slight variations sometimes. So, it is important to ensure that you’re using multiple resources just to make sure that every variation is covered.
The second way that we also do this is using the operators in a way. So that the terms that we’re using are on an open side. So, we use operators based on whatever database we’re using. The operators are used in such a way as to cover more ground. So, if the compound name, I will not use it as it is. I will leave some space in the terms so that broader coverage is attained.
Last and most important way, is to go through all patent and the non-patent literature in the domain. And see how your compound or the excipient maybe mentioned or disclosed in a certain document. And those variations can be then taken up and used in our term-sets. It ensures that every variation that is possible in the prior-art search in pharmaceutical domain is covered.
Are Structure Based Searches or Analysis Important in an FTO search in Pharmaceutical Domain?
Ram Tenneti Speaking – Now, there is another important question that has come from the audience. Pooja, I would like you to answer this.
Are structure based searches or analysis important in an FTO when you are talking about the pharmaceutical formulation? The reason why I ask you since the focus of Pharma formulations is not usually on the chemical compound itself.
Pooja Chhikara Speaking – Yes, the structure based search is very important in this case or an FTO search. The reason being – first, it is the chemical agent that is most relevant for a drug formulation. It is the active agent of that formulation. Further, second is a majority of patents cover those chemical agents in the form of MARKUSH structures. So, to check the infringement in such patents, it is important to perform structure based searches in FTO. It ensures nothing is missed out and so that the patents can be captured in structure search. It will be those which missed out in the keyword based search.
When Patents are Considered Relevant and Non-relevant While Searching?
Ram Tenneti Speaking – I have some other interesting questions from the audience. Harsha, she can answer me. In case, the technology comprises of a composition for a specific indication. Are patents mentioning all of the indications considered non-relevant?
Harsha Agarwal Speaking – I think a lot of information about this specific question was covered by Pooja. Where we discuss that in cases where the independent claim in itself discloses another indication. Therein cases, the patent might not be considered relevant. It is because as you said and as the question mentioned that the indication of the target formulation is known.
So, in that case, if the patent is disclosing some other indication in the independent claim itself, then in that case, from an FTO perspective, the patent would not be considered relevant, but, again, there may come a situation where the indication is disclosing the dependent claims, or the patent specification and not in the claim itself. Hence, in that case, in an FTO search, it would not impact the relevance. It is because broadly the patent would be disclosing the formulation that we are looking for. And thus in that case, the patent, again, might become relevant. Thus, depending on where the indication is being disclosed in the patent, the relevance is usually decided.
When Excipients are Considered Relevant While Searching?
Ram Tenneti Speaking–
Now, over to Pooja – I have a quick question for you, and then, probably, we’ll wrap up the session. It is because we’re nearing time as well. Pooja, you mentioned that excipients are not usually considered important while evaluating the relevance. Now, if there is a case where our technology is a composition that does not mention any excipients. But we come across a patent with the composition comprising the same active agents but specific excipients that are affecting the efficacy of the composition. What would be the relevance of such a patent?
Pooja –
Okay, well Ram. In this case, the patent with the excipient would be considered non-relevant. The reason is, excipient is acting as one of the active agents by affecting the overall efficacy of the composition. So, the excipient is playing a major role in this composition here. So, that’s why it would be considered non-relevant as the original drug formulation does not have any excipient. And we had covered this point in the PPT as well wherein excipients I’ve taken into consideration for analysis. It was when they are acting as active agents and playing an important role in the drug formulation.
Ram Tenneti Speaking –
All right, I think those are the questions that I have come across from the audience. And I feel these are some of the important ones that needed to be covered.
Is there anything else, Harsha or Pooja, you would like to add?
Harsha Agarwal Speaking –
No, Ram! I think we have presented from our side. If there are any more questions, then we would be happy to answer them.
Ram Tenneti Speaking –
Well, I see those are the important questions that we have already answered to.
So, well, let me quickly say thank you to everyone who has been today here. This has been a wonderful session, and I’m sure all the listeners have great takeaways from this session. And can use several of these pointers when working on searching and analysis in their own respective fields.
I’m not able to cover some of the questions, because I don’t think they are much relevant. Further, the things that you are asking were clearly visible in the presentation. But, in case, if you feel, do write up, and we’ll be more than happy to get back to you. You can reach us at [email protected] or you can also reach me directly. I want to say, big thank you to all our listeners who helped us start on time and finish as well.
We really, really appreciate it and thank you very much. Please take care of yourselves and your families. Stay aware!
Have a great day ahead. Thank you!
Submit Your Information to watch the Webinar Video:
"*" indicates required fields




