Current Diagnostics, Therapies, and Vaccines for Coronavirus – Webinar
Current Diagnostics, Therapies, and Vaccines for Coronavirus
Key Points Covered in this Webinar Session:
- Key findings from our report:
- Therapies in pipeline
- Current partnerships
- Diagnostic Techniques
- Demonstration of Relecura Tech Explorer for COVID-19
Table of Contents
Speakers
Dr. George Koomullil, Founder & CEO, Relecura
Harsha Agarwal, Project Manager – Life Sciences and Chemistry, Sagacious IP.
Ram Tenneti, EVP, Sagacious IP
Submit Your Information to watch the Webinar Video:
"*" indicates required fields
More details about this webinar:
On December 31, 2019, several cases of pneumonia in Wuhan City, Hubei Province of China were reported to the World Health Organization (WHO). The novel virus, now known as SARS-CoV-2, has since spread across China and to other countries and territories. WHO has named the disease caused by this novel coronavirus: COVID-19. In the current world crisis where Coronavirus has been declared as Pandemic by WHO with 935,817 cases and 47,208 deaths (as of on April 2nd), it is imperative to find a solution. Researchers are working tirelessly to stop and treat it.
Webinar Transcript:
Ram Tenneti Speaking – Welcome everyone to the webinar. Good morning, good afternoon, good evening! At wherever the part of the world that you are listening from. This is Ram Tenneti. I am the executive vice president at Sagacious IP, signing in from India to welcome you all to our webinar today on the topic “Current Diagnostics, Therapies, and Vaccines for Coronavirus.”
Now, before I go on to introduce this topic and the esteemed speakers on our session today. I am delighted to welcome all the participants from different countries. We have an entire world mix up here. There are people from Australia, Canada, France, India, Thailand, United Kingdom, and the United States as well. As I always say that your participation is a wonderful encouragement to the efforts and attempt that we are making to raise awareness and spread knowledge that has been honed by Sagacious over several years of working with inventors, research and development organizations, IP departments, and IP law practices.
Now, to take things ahead, let me quickly introduce our first speaker. She is the project manager with Life Sciences and Chemistry division at Sagacious IP. Her name is Harsha Agarwal. To give you a quick understanding of who Sagacious IP is, we are a 350 plus strong Patent and IP professionals in the business of patent search for over 14 years.
Welcome to the webinar Harsha!
Harsha Agarwal Speaking – Thanks Ram, thanks for having me on the webinar.
Ram Tenneti Speaking – Thank you, thank you!
Let me also introduce to you another of our esteemed presenters today. George Koomullil, he is the founder and CEO of Relecura. George has over 25 years of experience in research and development and with IP industry as well. To give you a quick understanding of what Relecura does, it’s basically a software product company that was founded in 2009, and Relecura offers artificial intelligence platform on patents and scientific literature. Welcome to the webinar George!
George Koomullil Speaking – Thank you, Ram, for having me on the program.
Ram Tennati Speaking – Wonderful, thank you, George! Thank you George and Harsha! Guys, before we start off with the presentation today, let me ask our presenters for their initial remarks on the current crisis of COVID-19 and how patent literature is relevant in these times. Why don’t we start with Harsha?
Harsha Agarwal Speaking – Yeah, sure, Ram!
Currently, we’re facing a global crisis that is affecting us in multiple ways. This is not just a health crisis but a political, economic, and a social one. In this time of crisis, scientific advancement is our only aid. Fortunately, we are seeing a global scientific collaboration unlike any in history. We are witnessing a one-of-a-kind event where scientific experts from every corner of the world are focused urgently on one single problem, and, more importantly, this collaboration is happening very openly. Multiple research platforms, universities, corporate, and research group have come forward to collaborate in finding a cure and a vaccine against this pandemic, and, in this time, both research literature and protected patent literature are crucial in development of a drug. When we talk about the pharma industry, especially, in our pharma industry, patent literature is a goldmine of information. Drug patents specifically have information ranging from their composition, their dosage, efficacy, and even the target of the drug.
The mining of this information and using this information are very significant when trying to identify new potential candidates for drugs or vaccine or also developing the identified candidates further as treatments. Thus, we can say that patent literature, especially in the field of pharma, and overall research literature is very crucial in this time of crisis to help us fight the pandemic that is COVID-19.
Ram Tennati Speaking – Great! That’s very insightful, Harsha. Thank you, thank you for your initial remarks. George, would you be kind to share your initial remarks please?
George Koomullil Speaking – Yeah, sure, sure, Ram!
Okay, so, if you look at, the COVID-19 has already impacted almost everyone’s life globally, and when there is so much uncertainty, it is good to have a look at where we are and what the potential outcome could be. I can think of three different ways this can pan out.
One is that we will come up with a drug or vaccine soon to treat it. We will contain the virus so that it will not spread anymore, or we will move towards the herd immunity to contain it. That means that enough number of people gets the disease, gets immune and stops the transmission. The first one would be, almost, like in the hands of the scientific community. The second is almost impossible as it spreads fast and it’s already reached everywhere in the world. The third scenario is the worst case and is what the governments are actually planning, but at the same time you should note that the main reason for the current crisis is not the virus is being too deadly but that our healthcare system is not able to handle the scale of new patients it can bring. If we can control the rate or transmission, do social distancing, for example, or increase the capacity of the healthcare system, everyone will have access to proper treatment and the prices will be under control until the drug or vaccine comes out.
Now, coming to the first scenario, we can presume that when the scientific community discovers a drug, it would be based on or built on the existing technical knowledge. If you combine that with the frequently quarter statistics, that 80% of technical information is found only in patents which is especially true for pharmaceutical companies because their value is mostly through their patents, so it is clear that the basis for the solution lies somewhere within the patent literature and the researchers and scientists need to use it to come up with a solution.
Introduction to Current Diagnostics, Therapies, and Vaccines for Coronavirus
Ram Tennati Speaking – That’s very insightful, George. Thank you, thank you for those quick initial remarks. That really sets the context for today’s webinar – Thank you Harsha and George for setting the context.
Now, before we move ahead, I would like to invite all our listeners. Let us now get started with the main part of the presentation and for that let me invite Harsha to take us through and help us understand “Current Diagnostics, Therapies, and Vaccines for Coronavirus.”
Over to you, Harsha!
Harsha Agarwal Speaking – Thanks Ram!
A very warm welcome to all the participants on my behalf! My name is Harsha Agarwal and I’m working as a project manager with sagacious IP. I have been with the company for four years and we are involved in identifying patents, patent landscapes, doing market research, literature research and all sorts of research relating to the life sciences and the chemistry domain and today we will be talking about what this COVID-19 pandemic is and how it is affecting us worldwide also we will be talking about what options are available as treatments or diagnostics as of now and the therapies or the vaccines that are currently in the pipeline and that we may be expecting soon.
Let us start with the presentation on “Current Diagnostics Therapies and Vaccines for Coronavirus.” If we talk about the COVID-19 pandemic, the virus that is responsible for the COVID-19 pandemic called the SARS-Coronavirus-2 virus is not a new virus. Researchers first isolated the coronavirus in 1937 in birds, and even then it had the ability to devastate poultry stocks. However, the first evidence of human coronavirus was found in 1960s in the noses of people with common cold. The Wuhan 2019 outbreak, now, is happening because of the SARS-Coronavirus-2 which is a new strain of the same beta coronaviruses.
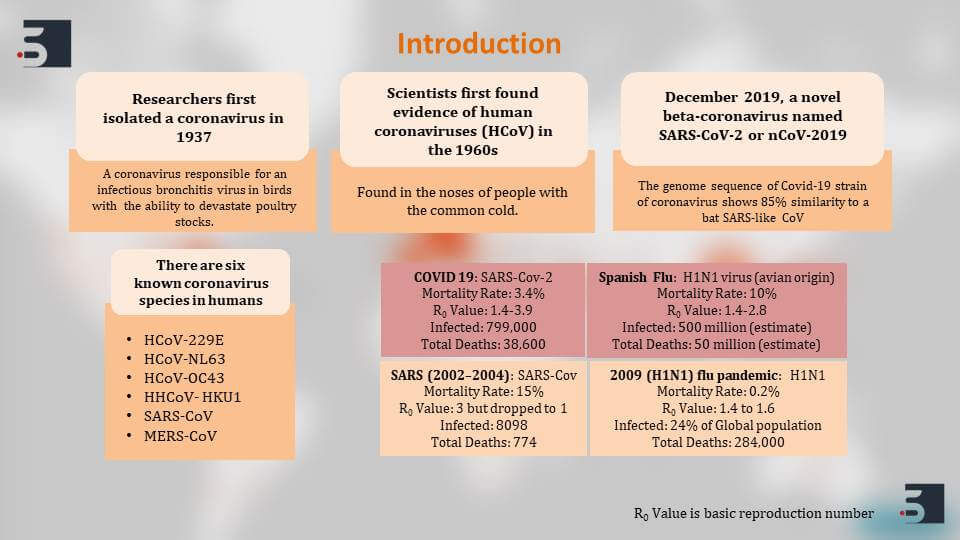
Till now, there are six known coronavirus species that are affecting humans – the first four species that we can see here the HCoV-229E, the NL63, the OC43, and the HKU1. All these four viruses show very mild symptoms in humans like common cold symptoms. However the SARS coronavirus and the MERS coronavirus, both cause respiratory syndrome and can be quite extreme.
If we talk about and compare the COVID-19 pandemic with the similar respiratory outbreaks in history, it is almost comparable to the Spanish flu. The Spanish flu that happened in 1918 killed around 50 million people worldwide and affected 500 million people. The mortality rate of the Spanish flu was much more higher than COVID-19, but COVID-19 is still as deadly as the Spanish flu because of three factors that I think are responsible for its fatality.
The first factor is its high R-naught value. The R-naught value – when we talk about is the basic reproduction number. So, if I say that R-naught value of COVID-19 is 2, it means that one infected person will infect two more people.
The second reason is the long incubation period of the COVID-19 virus, but the third and the most important reason for COVID-19 being so deadly is the fact that affected people show mild or very less symptoms, and thus making it very difficult to isolate cases and stopping the spread. Then, we compare the SARS outbreak that happened in 2002 – The R-naught value initially was 3, meaning that one infected person could infect three people, but because SARS showed very extreme symptoms in people, it was easy to isolate them and thus the R-naught value quickly dropped to one thus stopping the outbreak. In COVID-19, this is not been very easily possible because the symptoms are not clearly visible.
Structure of Coronavirus to Identify Diagnostics, Therapies, and Vaccines for Coronavirus
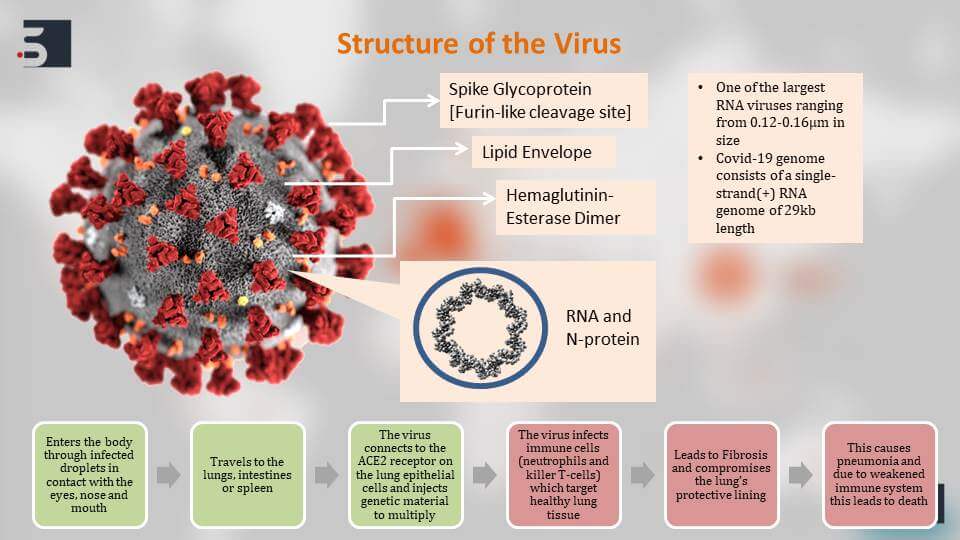
If we take a deeper dive into the structure of the virus and how it affects the human body, we will see here that the virus has a lipid envelope and it has these specific spike glycoproteins which are specific to these virus and help in its virulence. These spiked glycoproteins have furin-like cleavage site. Furin is an enzyme that is commonly found in humans and thus the virus can very easily bind to human cells. When the virus enters the body through infected droplets, it travels to the lungs intestines and spleen where it can easily bind to the ACE2 receptor on the lung epithelial cells. It further infects the immune cells leads to fibrosis and also weakens our immunity. This weakening of the immunity leads to pneumonia can lead to organ failure and eventually to death.
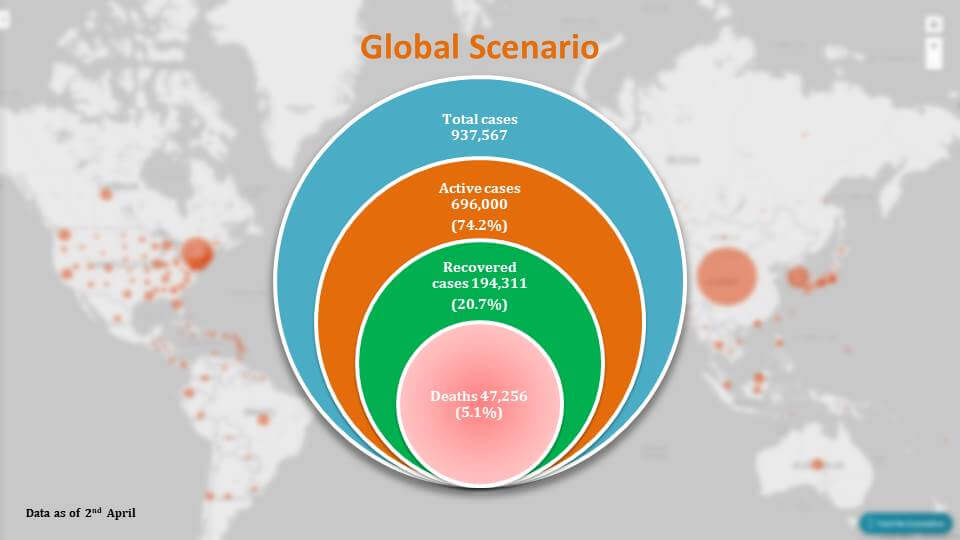
Global Scenario
Talking about death, if we go see the global scenario, we can see that the devastation it is causing is deadly. There are around 937,000 cases currently more than those cases because this data is of 2nd April in the morning, and there have been 472,56 deaths that is five point one percent of deaths so far only 20% of the cases have been recovered.
What are the Current Diagnostics, Therapies, and Vaccines for Coronavirus?
Let’s now look into the current diagnostics, therapies, and vaccines for coronavirus and see what options we have to fight the COVID-19 pandemic. The current treatments that haven’t been approved but are on the list of WHO are mentioned here:
Treatments for Coronavirus
For example, Remdesivir by Gilead Pharmaceuticals, Favilavir by HISUN Pharmaceuticals, or Kaletra by AbbVie – these are all being tested in people that are already affected with the virus and a lot of them have shown positive results against the virus there have been confirmed recovery cases – also, in case of a combination of Ganovo & Ritonavir by Ascletis Pharma of China.
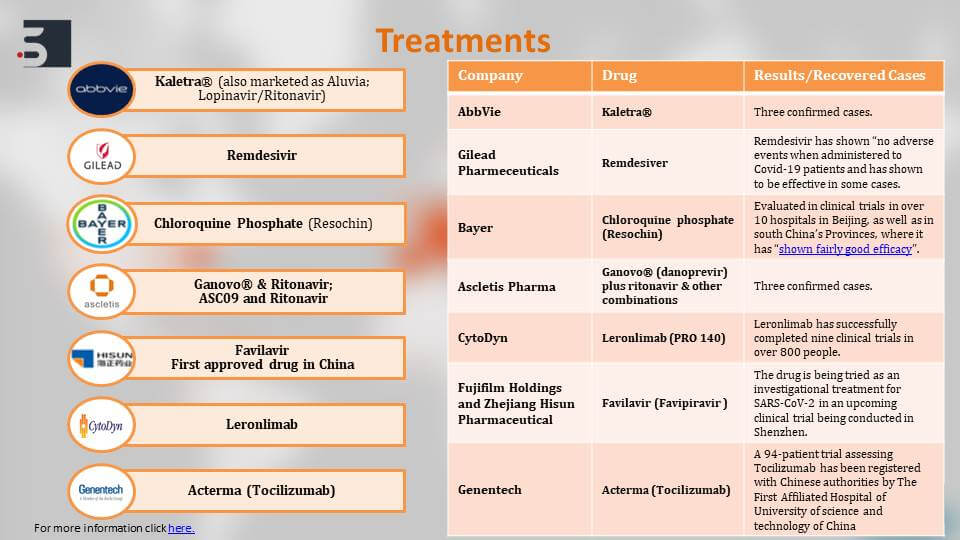
Similarly, there have been recovery cases using Kaletra. We’ll talk more about these treatments and their clinical trials in the further slides.
Diagnostics for Coronavirus
We talk about the diagnostics that are that exist currently. They can be broadly classified into Molecular Assays and ImmunoAssays.
There are numerous kits that are available right now for detecting COVID-19, but majority of these kits are only applicable in a research lab setting. Although the ones commercialized mentioned here are the one that are being used in the human population in real life.
For example, the 1drop Inc kit or the AusDiagnostics kit is being used to detect COVID-19 in the affected population. These Molecular Assays depend on PCR techniques to identify the markers of the COVID-19 in the gene.
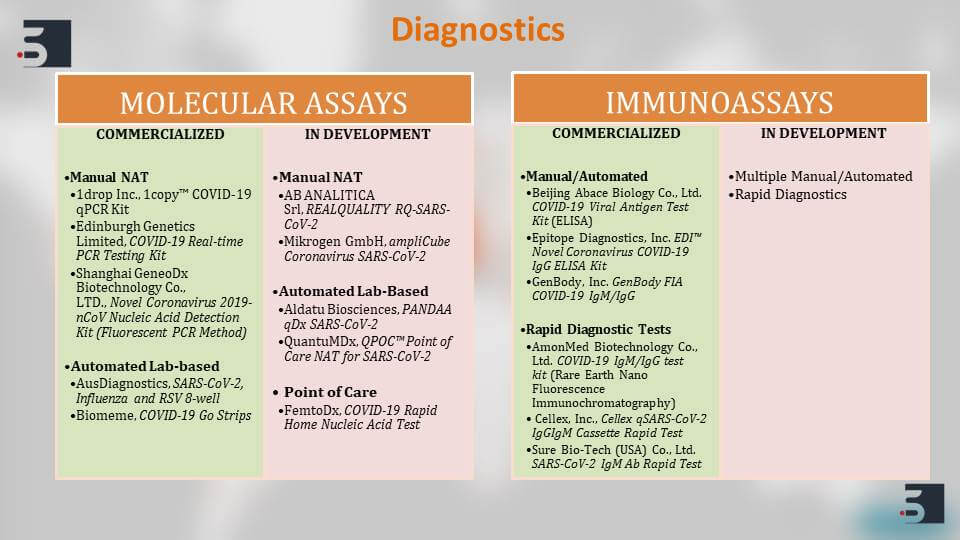
The second kind of Assays that are available are the Immunoassays or most commonly known as the ELISA, and we also have some commercial Assays of this kind which are being used currently and there are multiple ones that are being developed further.
Apart from the traditional diagnostic Assays, Companies like Roche and Abbott have come forward with these new diagnostic Assays that can detect the COVID-19 in human population very quickly. If we talk about quickly, Abbott’s test, which approved the emergency authorization for its use, is very rapid and it’s portable. It can detect and give a positive result for coronavirus within 5 minutes and the negative results in 30 minutes.
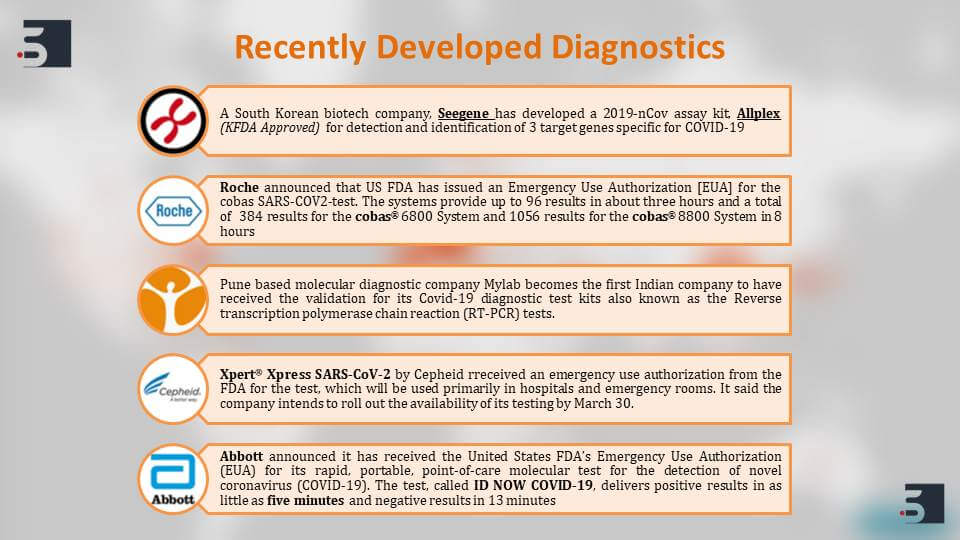
Roche’s kit can give the results in about 3 hours. Even an Indian company called Mylab has come up with this diagnostic kit which uses RT-PCR, and they’ve claimed that this kit is cheaper as compared to already existing kits thus helping the Indian government make this process easier.
Therapies in Pipeline for Coronavirus
Now, we will move forward and discuss about therapies that are in the pipeline from our webinar topic diagnostics, therapies, and vaccines for coronavirus, and we’ll talk about the options that we’ve discussed initially.
The drugs and vaccines that are currently in pipeline – there are more than 66 programs that are currently working on them and the entire process of development can be divided into three different approaches.
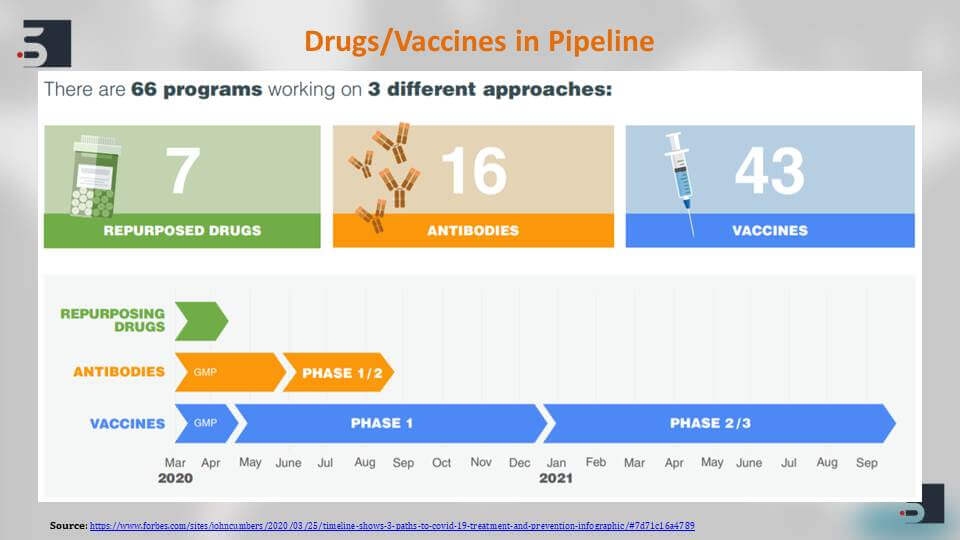
The first is the repurposed drugs, the second is the antibodies or other proteins, and third is a vaccine. Repurposed drugs are basically those drugs, which are already known to be effective against viruses in humans, have shown antiviral effects, and now they are being tested against the SARS coronavirus 2 species, and the benefit of using repurposed drug is the fact that because they have already known to be effective and safe in humans, the approval process for this is very short as compared to the vaccine. When started from scratch, it can take at least a year to be tested and come to the aid of human population.
Initially, our first line of defence against the COVID-19 pandemic is those repurposed drugs that we initially you also talked about. The Remdesivir, for example, by Gilead or the Acterma by Genentech – these are known drugs against, for example, Ebola or HIV, and there are different kinds of targets and mechanism of actions. Remdesivir is a nucleotide product while Ganovo & Ritonavir are Protease inhibitors.
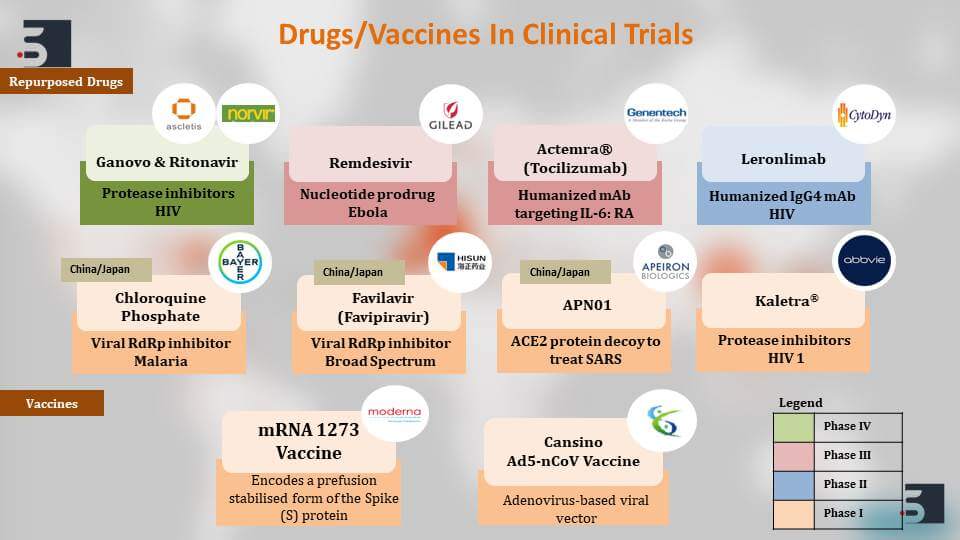
Similarly, there are antibodies that target specific interleukins that can help in stopping the infection. There are certain drugs like Favilavir and Chloroquine Phosphate that are in trials in China and Japan in their phase 1 trials and are shown to be effective against the virus.
We talk about the vaccines. There are two candidate vaccines that have already entered the phase one of their human testing which is a great feat if we talk about the development of a vaccine. The Moderna mRNA 1273 vaccine – it encodes a profusion stabilized form of the Spike protein. The S protein that we saw on the surface of the virus, and another one is Cansino biologics vaccine. It is an adenovirus based vector that is now in its human trial phases and we hope to see them moving forward to their advanced stages very soon.
There are also numerous potential drugs which are in their preclinical stages or animal testing stages and are showing good effects against the virus. To list a few we have included Sanofi’s Kevzara or Biocryst’s Galidesivir. These, again, have different mechanism of actions. Some are polymerase inhibitors and some are antibodies, but they all can affect in stopping the infection of the virus.
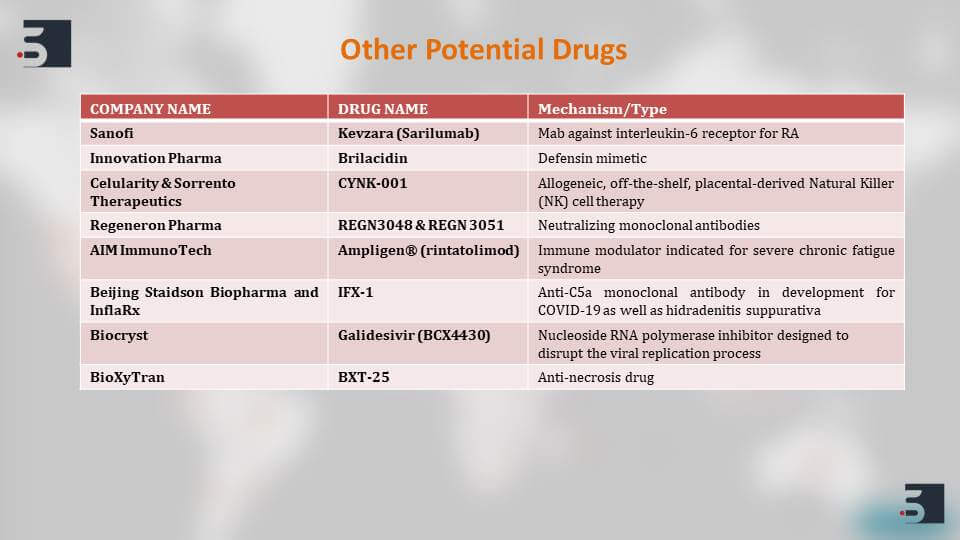
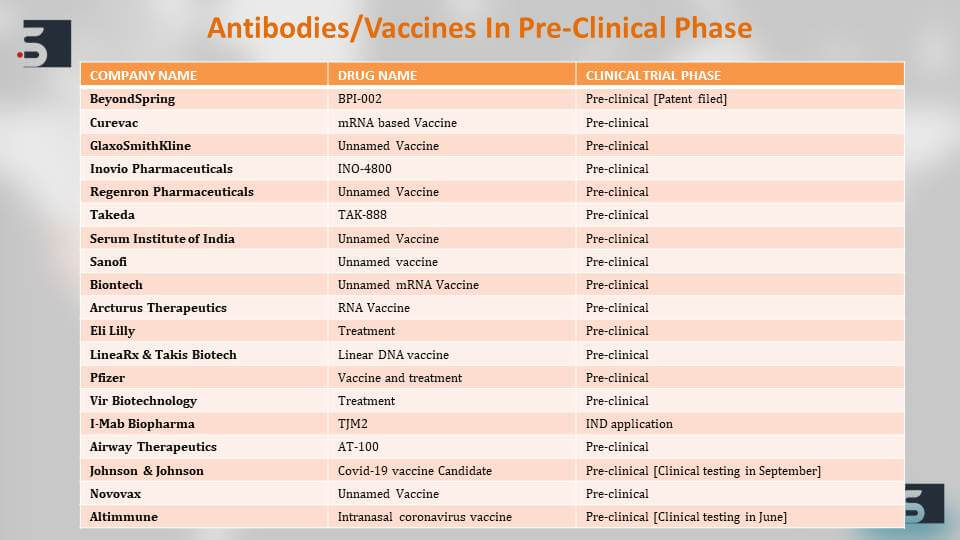
Apart from the two vaccines that we saw are in the clinical trial phases, there are multiple vaccine candidates that are in their preclinical phases. The big players in the pharma industry have also come forward like we can see GSK, Johnson & Johnson, and Sanofi. They have all come up announced their vaccine candidates which are in their preclinical stages and a majority of them have also received fast-track approval from the FDA for clinical testing and we might be expecting their clinical trials to start as early as June.
The most important thing that is happening in the scientific community right now is the collaboration between the academia and the corporate to find diagnostics, therapies and vaccines for coronavirus. We can see that there are multiple vaccines that are under collaborative development and this collaboration is happening.
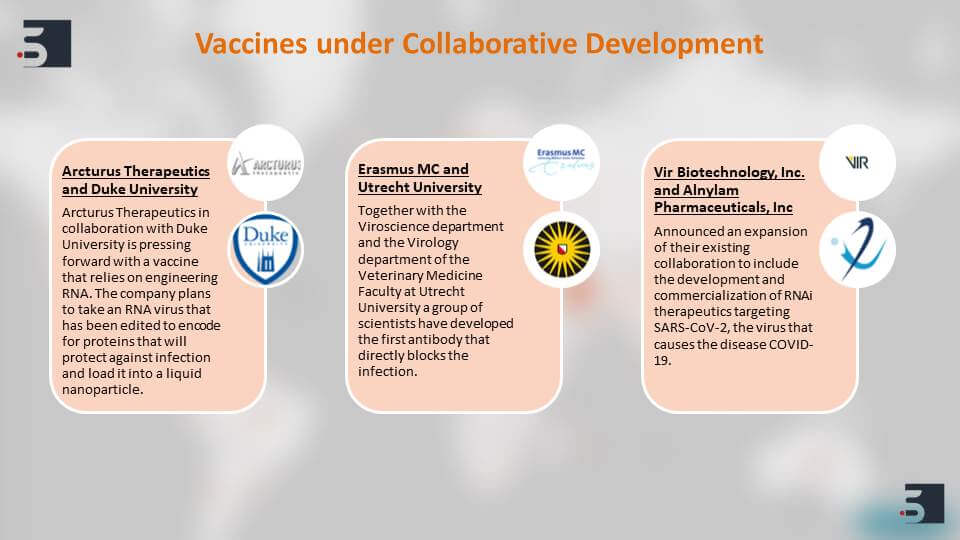
For example between, Duke University and Arcturus Therapeutics and Utrecht University and Erasmus MC – the Duke University collaboration has come up with a vaccine that relies on engineering RNA and the Utrecht university has found and developed an antibody that blocks infection directly.
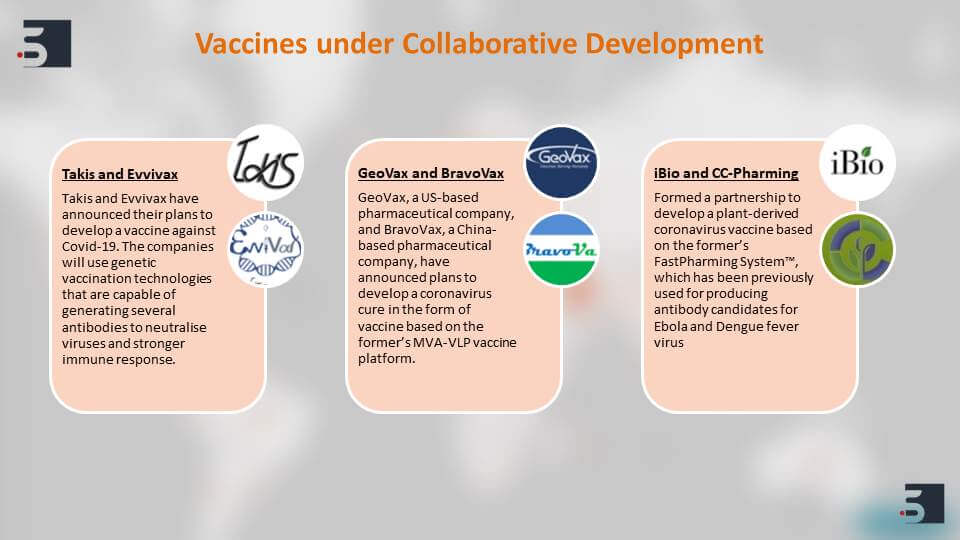
Similarly, we can see this under this time of crisis the collaboration is appreciable and there are multiple candidates that are in their preclinical stages or animal stages or testing stages and will soon move forward to their advanced stages.
There are certain diagnostic kits also that are in the pipeline currently, and for example IIT Delhi has come up with a probe-free method, and they claim that it can detect the virus very quickly it is currently under validation. Everly well is a company that provides at-home lab testing kits, and they have also announced that they’re producing at-home tests for COVID-19.

All of these efforts in developing these diagnostics whether be cheaper options or quicker options or options that provide at-home lab testing will surely help in reducing the burden of the healthcare system.

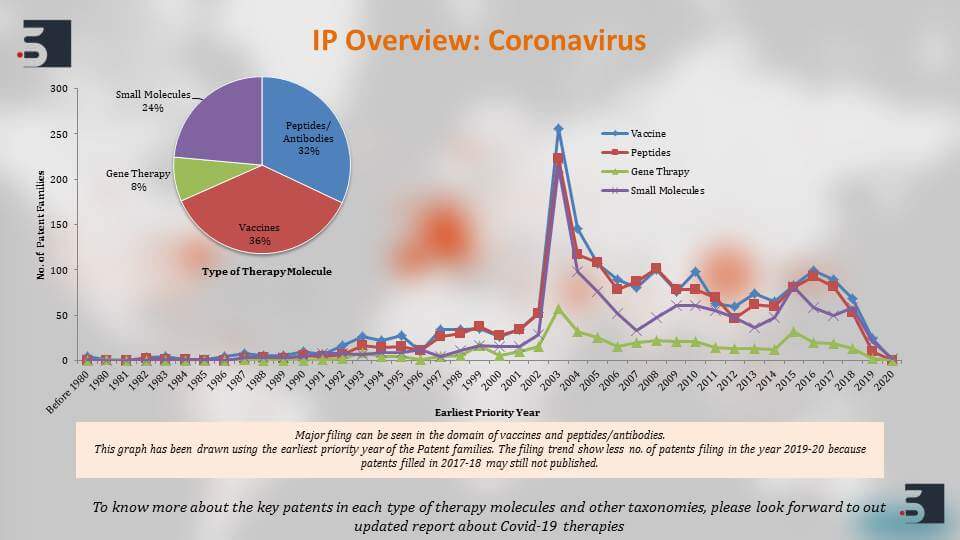
We, at Sagacious IP, are having our expertise in the patent area also performed a quick landscape to identify patents against the coronavirus. Moreover, we saw that there are around 4300 patent families if not more that are relating to the coronavirus and if we see the overview of these patent filings then there are around 68% of these patents that are focusing on the therapies while 32% of them are focusing on diagnostics.
Also, we can see that there is a spike in the patent filings, a major spike in the filings in 2003 and this is in direct correlation with the first SARS outbreak that happened in 2002, and the less number of patent filings in 2019 and 20 is because patents take around 18 months to publish but overall after the SARS outbreak although the patent filing decrease, there has been a steady filing activity that can be seen.
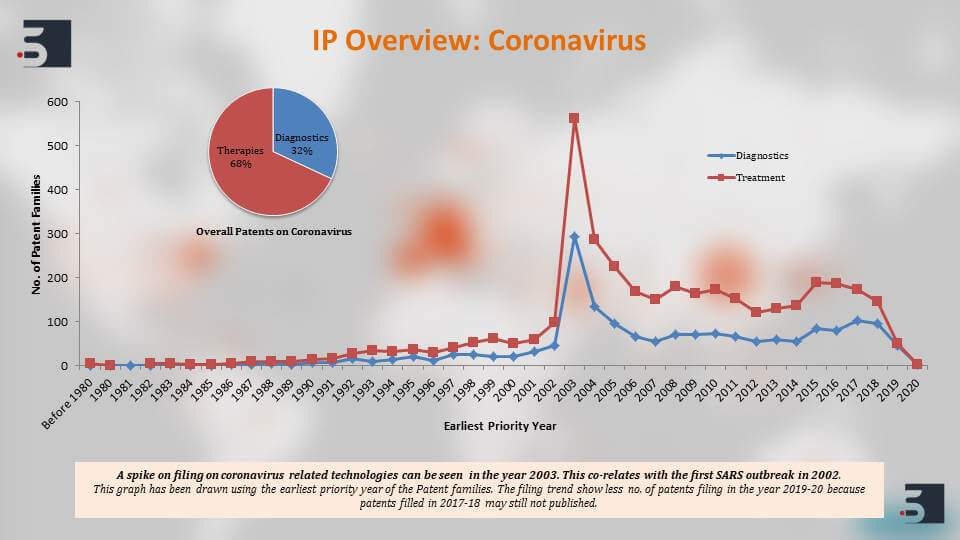
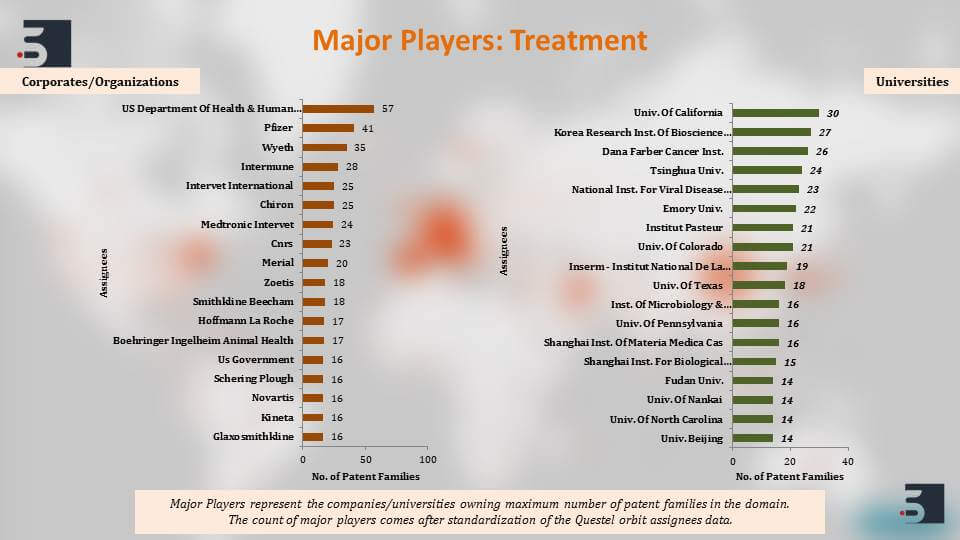
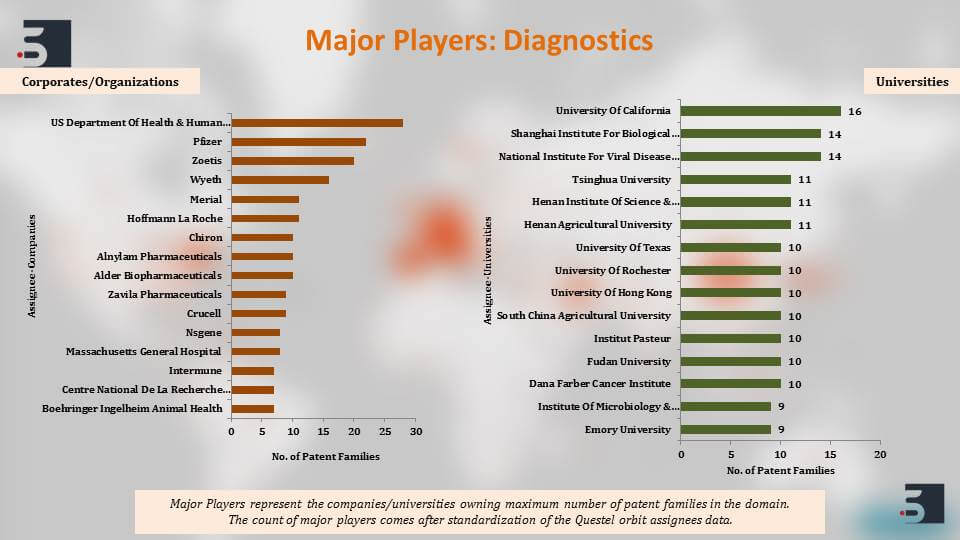
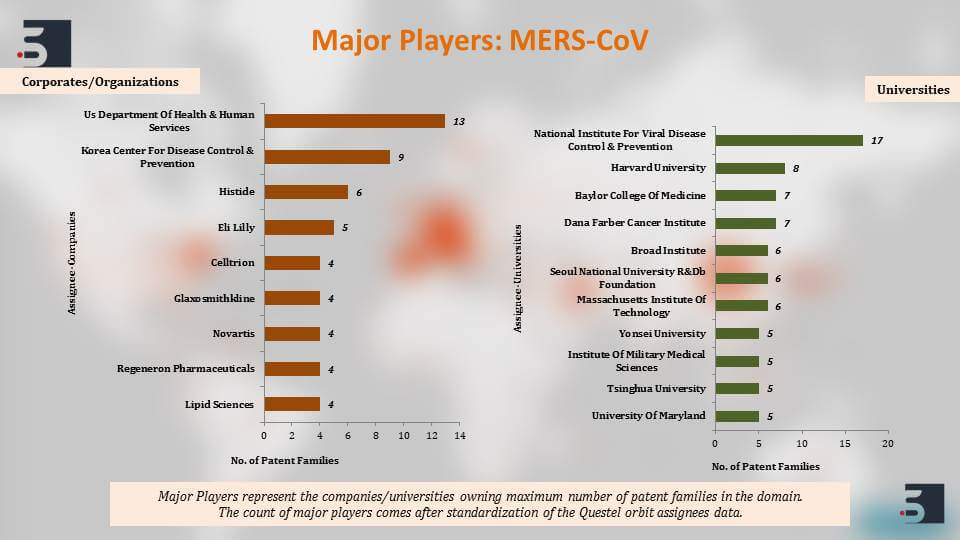
The major players that are filing in this domain of diagnostics, therapies and vaccines for coronavirus, the top names that come up are the US Department of Health and Human Services, University of California, Pfizer, Intermune, also Chinese Institute’s like Shanghai Institute for Biological Sciences and then further to clearly understand the patents against the coronavirus we segregated the patent filings based on the target of the drug or the vaccine and we saw that around 46% percent of the pavement filings were against SARS and 11% against MERS. Again, as you can see, the increase in filings happened and that is in direct correlation with their outbreaks.
The SARS one happened in 2002 and there were increase in filing in 2003, and the MERS outbreak that happened in 2014 and then we can see the increase in filings there.
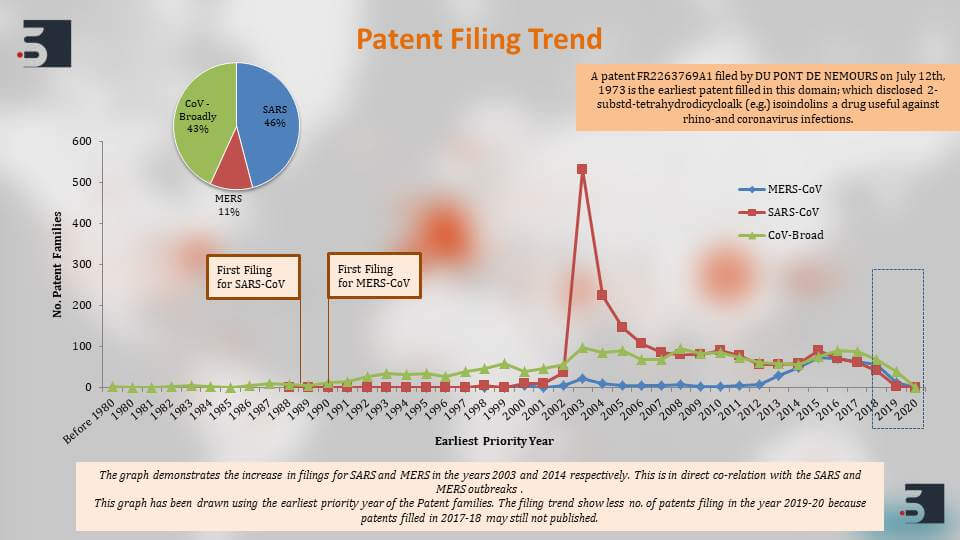
However, the first patent that was filed was DU PONT (DU PONT DE NEMOURS) in 1973, and it was a small molecule against the coronavirus and we can also see that there have been steady filings against coronaviruses in general. The broad patents that do not disclose what strain but generally talked about molecules or therapies against coronaviruses in general. There has been a steady filing in those in that area.
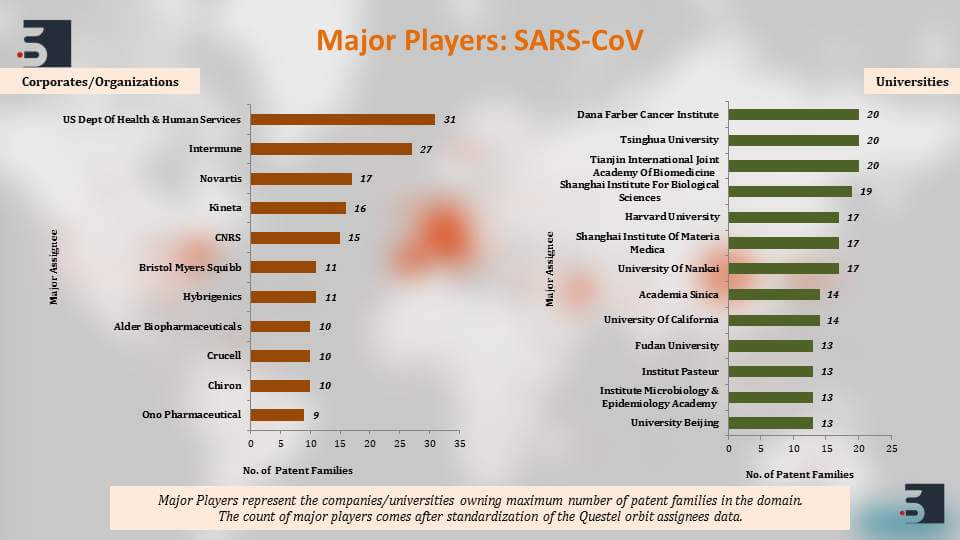
The major players if we see are the US Department of Health and Human Services, Intermune, Novartis, and also majority of a few Chinese universities. Harvard and Korea Center for Disease Control and Prevention are also filing prolifically in the domain.
Further, we decided to divide the patents as per the type of therapy molecule and we can see that 36% of these filings are about vaccines and so overall we can see that antibodies or biologics in general comprised majority of the patent filings. There are small molecules as well which comprise 24% of the filings.
To know more about the key patents in each type of therapy molecules and other taxonomies, please look forward to the updated report about COVID-19 diagnostics, therapies, and vaccines for coronavirus that we will be soon sharing on our handles and just this is to summarize what we’ve discussed today.
The pandemic is comparable to the Spanish flu, 1918 pandemic, because of its virulence and the fact that it shows mild or no symptoms and also its R-Naught value of average of 2.5.

There are multiple diagnostics, therapies, and vaccines for coronavirus that are being currently used and tested, and multiple drugs that are in the pipeline, a few of them are also in their very advanced stages. For example, the combination of Ganovo & Ritonavir in the phase four of trials and it is showing very good results.
Multiple vaccine candidates are in their preclinical stages and are being fast-tracked to their clinical stages. Overall the patent filings sped up in 2002, and we are expecting to see more filings now after the COVID-19 outbreak. That is all from my side.
Relecura and Tech Explorer
Ram Tenneti Speaking – Thank you, Harsha for that comprehensive overview. That was really an in-depth understanding of the entire situation and the pandemic as a whole. Now, let me ask George to take us through the Relecura platform and specifically walk us through Tech explorer and the taxonomy of the coronavirus based patents. George, over to you!
George Koomullil Speaking – Thank you Ram! Hello everyone! Welcome to the program.
I’m George Koomullil, founder of Relecura. So, we have a software product company based in the Bay Area. We have offices in Pleasanton and in Bangalore. We have a cloud platform that uses machine learning, AI and natural language processing for processing patents and scientific literature. Our platform and all the analytics tools are available free for coronavirus researchers and what I plan to do is that I’ll go through a few slides to give an overview of the platform and a few simple cases that are relevant to the research.
Let me start here. Here is an overview of our platform. To start with, on the left side you can see that these are the type of data that it contains. Patents, Scientific documents, assignments and some personal documents you can bring into the system and some litigation data, and here are some of the tools that are built on the platform. I’ll be going through the Tech Explorer part in more detail and also the web app and there are other ones as well.
Here are a few of the features like how to explore a topic and how to find the related topics, how to do a semantic search and retrieve relevant documents that you are interested in, and I will also show a dynamic taxonomy. Taxonomy is a hierarchical categorization of a list of documents and again we have global patent data so feel free to use – everything that is available there and some of the data visualization in it.
Here is a look of Tech Explorer. What I’m going to do is that I’m going to take you through tech-explorer through a live demo. I’m going to bring it up here. If you click on that, you would have got coronavirus. This is the topic that we started with. Now, you can see that on the left side there are different colour codes. These are all grouped differently. Now, let me take you through the list. If you look at you know there are concepts that are shown in one colour and those are all related to protein, and similarly, there are other colours, then you can look at you know different types of categorization that are there.
Now, I will go here and let me select another one for example. The idea is that you can start with any search and then go in two different directions or different paths that are of interest in your research.
I will take vaccine and explore. Now, the system is going to bring up research based on both selections coronavirus as well as vaccine. Again, it is further divided. You can go further and further. Now, if you go down. Scroll down. You will see the patents that are related to the search that you have done. So, it is very simple again. So, here is one from February 2020 and then you can click on that to see more details about that or you can click on the link to see, again, the details of the particular patent.
Now, if you’d like to see some scientific documents, you click on that again. Here are some of those. Now if you have any documents that you have kept in your own account, you can click on my documents and you will get it or go externally to see other ones that we are giving here. By the way, whatever we are giving here on external links, these are not on our servers. Basically, we are sending you to their servers and to get the results.
Now, coming back to our search, if you have your own documents, you can just go to my folder, and then upload your documents. When you upload the documents and submit it, the system will provide you a way to categorise those.
For example, I have a few documents over here, and then if I go to another one, you can see that it will automatically categorise that and the categorisations are all available you can use those categories so the models that are created by the system and you can use it for the next time when you bring more documents into the system.
Basically, what Tech Explorer provides is an easy way to do a search. For example, let me just start with a new search. I start with Tech Explorer. I will give ventilator, and then it gives different ways you can go further. Let me take oxygen concentrator here and then explore further. Now, you see all the things that are related to oxygen went over here. You can go further and further deep into your research and if you go down again, you will see patents related to that.
For example, here, this is from March 3rd – one on ambulatory generation of nitric oxide. This is one way we can use the system to get an overview of all the topics that are related to the area of your interest. So, this is one.
Now, I’m going to go through the other platform – the web app. This is taxonomy. Basically, what we did was that we have a list of patents here – 7338 documents and it is automatically categorised into subdomains of subcategories. Its dynamic in the sense that you can get a lot of details over here you can map multiple patent portfolios to that. So, let me just take you to this one. Once you create taxonomy, you can share it with others.
For example, so these are the 7338 documents. Now, if you click on that, you can see the details how the publication trend was and which are the top assignees. Again, that’s a part of Pfizer, and then we can look at any one of them. So you go through different subdomains or subcategories to see more details. So, this is one. Now, I’m going to show you – again this is a shared link and I can show you how to create one of them.
For example, here, I have the same taxonomy. I’m going to map multiple portfolios again. I have saved all these searches like Pfizer, NIH, and all of those. So, I update it and map the portfolios to that taxonomy.
I’m just showing you what are the different ways you can analyse a list or documents that you have or documents related to coronavirus. So, click on it – their trend, then go down. Pfizer has the maximum number and sub-domains as well.
In addition to that, you can do different ways of comparing those like you can compare, for example, Pfizer and drugs containing peptides here, and similarly you have different fields to search or use some of the visualization features available within Relecura.
The idea is that these concepts are based on our ontology at the back end of the system, so the size of the circle represents the number of documents with antigen in it and antigen, immunogenicity, and vaccine. These are all related together that’s why they are coming together as a cloud.
This way you can do various searches and you can categories the patents, and I will also show you search and what are the things that are available when you do search. In addition to that, you can just export these statistics. For example, if you export it, you will bring it up into an excel sheet.
Now, here are the documents related to your taxonomy and the portfolio’s that you pass through that. This way you can compare which company has in, what area or how many patents.
Similarly, you can compare the patent filings from different years. Let me just make it a little bigger. So, this way you can categorize your patens whenever you’re working on coronavirus, for example, like get into different types. This drug is containing active ingredients, and then there are antigens and antibodies. So, you can look at the distribution of the patents among different players in the field.
This is one, and another one is that if you want to look at what is the type of the topic that are declining or growing in this field. Instead of giving this, I can select for the last, for example, 2014, 2015, and 2016. You can have multiple, with each one is treated as one portfolio, and this time, we are doing for different years. Now, we have got the patents filed in this area from 2014 onwards. Now, you update it and mark the portfolios to the taxonomy.
Again, at the end of it, whenever you get the results, you can export it into an excel sheet and find out which are the areas where there are growth like a lot of growth happening in this particular patent filings in this area. I’m going to export it, or I can share it as well. When you export, this is one way you can look at whether it is an increasing trend or decreasing trend.
Okay, so, this just to give you an idea but know how the taxonomy was. Now, let me just go back to the original Search Interface. Here is the interface. Again, you can start with a search for a term or you can start with a company or you can go into the detailed platform with the different fields available for you to search, so you can search within title, abstract, or claim, or you can go all the way to what are the publication year. You can select those.
For example, if you just give “coronavirus” as in the virus and published it from, for example, 2010 and then just give that and do a search for diagnostics, therapies, and vaccines for coronavirus. This is just one term. There are different ways of increasing the number of documents by finding out related topics. We will give you training whenever you are doing that type of search. Here are the documents if you look at. The more details are available all the time. Now if you want to look at which are the companies or what are the technologies that they having subject-knowledge just for example.
This way you can fine-tune to any level of details that you want or you can read the patents itself. All of them are available or you can look at which are the ones that are growing or what are the things that are happening in the inventive course level. That’s a patent examiner’s classification boards. We can give that. So, by looking at if you want to find out what exactly it is, all the details are available here.
This is just to give you an overview of how Relecura platform can be used and what are the different types of features available in it and if you just want to create a classification very quickly, you can do that here as well. There’s a taxonomy field. You can decide how many nodes that you want and then you can keep increasing that. There are different types of different modes for creating different levels of details.
This is just so this is an overview of the platform again. All of these are available free to coronavirus researchers to do search for diagnostics, therapies, and vaccines for coronavirus and we provide full support whoever wants to try, and try using it for your research.
Now coming back to our slides so this is where you need to reach to request an account and support for that.
Ram, back to you!
Questions
What is the Role of IP Industry in the Research of Diagnostics, Therapies, and Vaccines for Coronavirus or Similar Outbreaks?
Ram Tenneti Speaking – Fantastic! Fantastic!
Thank you, George! Thank you for this elaborate coverage, and I must say that you have a fantastic tool. I see that it has a lot of functions and features that are very helpful to do searches, and I’m sure that people are going to come back to you.
Thank you again, and Harsha and George both of you have covered a lot of ground on this topic and we have definitely received a certain set of interesting questions which has got me thinking what kind of role do you guys think IP analysis or the IP industry as a whole place in the research of a treatment or a vaccine against the virus of this sort and why not let me ask Harsha to answer that.
Harsha Agarwal Speaking – Yeah, sure!
Ram, as we discussed in the very beginning of the webinar also that patents are goldmines of information especially when it comes to the pharma industries. A single drug patent can have a lot of information about the targets, and the composition, their efficacy, and all of those different kinds of information that is required for administration of a drug and also development of a drug.
Let me take an example here say somebody wants to develop a small molecule library against COVID-19 virus then a patent landscape search can help them identify what all small molecules have been used till date against similar viruses, for example, all the different strains of viruses, the SARS virus, the MERS virus, and also if we go into a broader aspect even the HIV or Ebola viruses.
We can use all of that patent landscape information, identify all of the small molecules that have been used against them, and develop a library of the small molecules, and using that library they can screen through the library and identify diagnostics, therapies, and vaccines for coronavirus. In that way, mining through the patent literature with the help of a patent landscape is very helpful in cutting down the time of identifying that small molecule library or similar compounds or the broader classes of compounds that can be used against the virus.
Same thing works with vaccines also when we talk about antibodies or the platforms for developing vaccines. There are multiple platforms for developing vaccines that are patented already. These platforms that already exist in our patent, which are being used for similar viruses, can be used to develop a vaccine against the coronavirus and that is why I think overall the patent literature is very important and can really help when it comes to developing a new vaccine or drug against coronavirus.
What is the Importance of Relecura Like Platforms for Researchers?
Ram Tenneti Speaking – Thank you for that answer, Harsha!
This is fascinating and let me quickly ask George a question. Do you think, George, the access to patent or scientific platforms, such as yours is, are very important for researchers.
George Koomullil Speaking – Yes, I certainly do, Ram. As I was mentioning earlier patents are critical for the discovery of diagnostics, therapies, and vaccines for Coronavirus. However, being legal documents, patents are legal documents. Patents are not always easy to read, and a lot of effort is required to understand what is there. Actually, more effort is put to making it legally strong than making the invention transparent to the reader. That is the case even for researchers who are experts in the field. So good platforms can help them get to the right information easily. They can get insights on each invention to the depth that they want to go into, and then it can bring multiple sources of information to bring it together and more importantly they can compare or connect different ideas or ideas from different patents together to assist in the process of discovery. Certainly, platforms would help them do all these things much faster than just reading the patent documents is real hard.
How Does Repurposing of Drugs Work?
Ram Tenneti Speaking – Okay, fair enough!Thank you, George for that answer, and guys there has been a very good set of interesting questions from the audience. Another good question that has come up is how does this repurposing of drugs work and can this information from patents help in the process? Harsha would you like to shed some light on that.
Harsha Agarwal Speaking – Yes, sure! Ram, we discussed during our presentation that our first line of defence against the current pandemic is actually repurposed drugs. When we talk about repurposed drugs, these are drugs or treatments that are already being used against similar viruses.
For example, the Remdesivir treatment – that was from Gilead pharmaceuticals was used against Ebola and had shown to be effective against Ebola and was also shown to be safe in humans.
So, now, they’ve taken up the same drug and now they’re using it against COVID-19. So, this is what is repurposing of drugs where we take an already existing drug which is known to be effective against viruses and use it for a new virus.
In our case, which is the COVID-19 virus, and the patent information again is very helpful here because most of these patents that we are repurposing are usually patent protected and thus all of their information can be found in one place in the patents and like if there is an expert who’s going through the patent of the molecule, they can identify a lot of important information about the molecule or the drug in question and identify how will it work and what dosage of it can work.
Similarly, patent information and mining of patent can also be helpful in identifying new candidates for repurposing like there was Remdesivir. We also would want to try more of candidates that would be repurposed for COVID-19. Again, these drugs that are already being patented – we can go through the patents and identify drugs which have similar targets in the virus.
For example, if we talk about the spike protein, if there are or protease inhibitors, if there are molecules small molecules or antibodies that are targeting protease inhibitors in already existing SARS coronavirus, then we can take the same drug and try it against the SARS coronavirus 2.
So, this way, the patent information helps in both ways – it helps in repurposing a drug that’s already being identified and also helps in identifying new candidates for repurposing.
How does Relecura help in Identifying the Diagnostics, Therapies, and Vaccines for Coronavirus?
Ram Tenneti Speaking – Great! Thank you, Harsha!
I’m sure the people asking this question must have got a great answer. Now moving ahead, there is another interesting question. I think it should be answered by George. George, they are asking how coronavirus researchers should make use of the Relecura platform. Now, that’s a very interesting question. I think I had the same question in mind as well.
George Koomullil Speaking – Okay, thank you, Ram!
Relecura platform has multiple tools in it. I would suggest that for those interested in exploring topics that are external to their own area of research but related to their area research. I would advise them to use Tech Explorer. It would lead them through different related paths and they can see how those fit into their own studies and their workflow is very simple and any anybody should be able to do that.
On the other hand, if they are interested to go deeper into topics, understand every detail related to an invention, then they should use the web app. The web app provides all sorts of details to use and that is being used by all experts in IP. It may be initially hard, but that is the one they should get into using for their area if they want to get into the details of their own area.
Another option is that, they can start with the Tech Explorer to identify a few relevant areas to dig deeper and then switch over to the full platform and again as I told earlier we will provide full support to anyone interested in using the platform and if the researchers actually have their own personal collection or technical articles, they can import those into Tech Explorer or to the web app and to get it automatically classify and manage their personal documents better and then those would be available to them whenever they do search in Tech Explorer so that when they do an exploration they can see what are the patents out there, what are the scientific documents out there, and what are the documents that they have in their own personal collection of data. All of them will be available to them together.
How Patented Repurposed Drugs Will Be Affected When They Come in Market?
Ram Tenneti Speaking – All right! That’s fantastic. Thank you, George, and are these drugs in the late stages of trials protected by patents and if yes, will that affect the prices of the drugs when they come into the market, Harsha?
Harsha Agarwal Speaking – Yes, most of the drugs that we’ve seen that were in the advanced stages of the trials were repurposed drugs as we talked about them. These drugs, mostly, are protected by patents, but when we talk about pharmaceutical patents, mostly, the patents are restricted by their uses and this is not applicable to all the patents.
There are also patents that claim only the compound and this can prevent anybody from using it for anything, but a lot of drugs or vaccines are also restricted to the diseases or the viruses that they are targeting. In that case, a few of them that are restricted already with the use of virus might not actually hinder the drug being used against COVID-19. It would not have any claims against the use of the drug for COVID-19, but also even if we don’t talk about these specific drugs or the claims, overall the government has also announced throughout the world – the governments have announced that the development of drugs or vaccines against COVID-19 virus will not be used like the companies or the corporates that come up with these drugs will not have the exclusive rights to produce them, and there will be provisions like compulsory licensing where the government basically forces the corporate to give the license to different manufacturers, and thus so as to maintain prices of the drugs which are affordable and, also, so that the production is at a capacity so that the drug is available to the majority of the population.
In my personal opinion, I do not think that patents in the case of COVID-19 will be hindering the availability of the drugs or vaccines to anybody as of now because of the pandemic and the crisis that we are in and the collaboration that we’re seeing right now.
How Can IP and Technology Platforms Support Researchers in the Future?
Ram Tenneti Speaking – Fair enough! Thank you, Harsha, for that. Now another set of interesting question that has come up here is now we all know that the entire world is moving on the technology front and the things are coming up based on artificial intelligence in specific to machine learning.
So, one interesting question we have, George, for you is that how can IP and technology platforms better support the researchers in the future?
George Koomullil Speaking – IP and the technology platforms have evolved in the last few years from regular search engines with the data and analytics into visualizations and automation of processes and workflows. For most of us not working in that area, all information on coronavirus might look too complex to grasp and this is the case even for the coronavirus researches. When they just get out of their area of specialization even if that particular information or knowledge or invention is very relevant to their own research, it would be very difficult for them to get it or it is too complex for them to get it.
Providing insights from different contexts that anyone can understand is something that the platform should provide next. There are lot of AI algorithms out there like if you want to look at a document from one context versus the other context, you should be able to that. That should come next.
Actually, more than that, it should be followed by another level of insights where the platform should have an ability to combine or connect related inventions and related knowledge, and actually that is what is going on in the brains of all researchers when they read multiple documents and then they try to combine, make sense out of that. That is what is happening and I believe that that is what the platform should provide or give in the coming years and that will certainly help with the discovery process of a drug or a vaccine or other solutions that are necessary in the future.
Ram Tenneti Speaking – Fair enough! Thank you, George! Thank you for that! Great sort of questions and answers from the presenters, I am sure this has been a wonderful session for all of you.
We are running short on time. There are a lot of other things that we can discuss, but unfortunately one hour is what we kept it for. I am sure our listeners had great takeaways from this session, and we shall be able to use several of these pointers. Please ask them to drop us an e-mail at [email protected].
I want to extend a very big thank you for all our listeners who have helped this to start on time and finish on time as well. We really appreciate that and thank you very much for joining the webinar “Current Diagnostics Therapies and Vaccines for Coronavirus.”
Have a great day ahead!
Submit Your Information to watch the Webinar Video:
"*" indicates required fields




